Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial
- PMID: 35796097
- PMCID: PMC9336498
- DOI: 10.1080/22221751.2022.2098059
Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial
Abstract
ABSTRACTBackground: In vitro studies have shown that several oral antiseptics have virucidal activity against SARS-CoV-2. Thus, mouthwashes have been proposed as an easy to implement strategy to reduce viral transmission. However, there are no data measuring SARS-CoV-2 viability after mouthwashes in vivo.
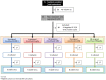
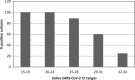
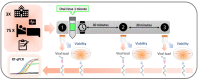
Methods: In this randomized double-blind, five-parallel-group, placebo-controlled clinical trial, SARS-CoV-2 salivary viral load (by quantitative PCR) and its infectious capacity (incubating saliva in cell cultures) have been evaluated before and after four different antiseptic mouthwashes and placebo in 54 COVID-19 patients.
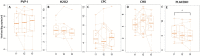
Results: Contrary to in vitro evidence, salivary viral load was not affected by any of the four tested mouthwashes. Viral culture indicated that cetylpyridinium chloride (CPC) significantly reduced viral infectivity, but only at 1-hour post-mouthwash.
Conclusion: These results indicate that some of the mouthwashes currently used to reduce viral infectivity are not efficient in vivo and, furthermore, that this effect is not immediate, generating a false sense of security.Trial registration: ClinicalTrials.gov identifier: NCT04707742..
Keywords: COVID-19; SARS-CoV-2; infectivity; mouthwash; saliva.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Worldometer . Coronavirus Cases. 2022; [cited May 27 2022]. Available from: https://www.worldometers.info/coronavirus/.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
