Analysis of Outcomes Associated With Outpatient Management of Nonoperatively Treated Patients With Appendicitis
- PMID: 35796152
- PMCID: PMC9250049
- DOI: 10.1001/jamanetworkopen.2022.20039
Analysis of Outcomes Associated With Outpatient Management of Nonoperatively Treated Patients With Appendicitis
Abstract
Importance: In the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial, which found antibiotics to be noninferior, approximately half of participants randomized to receive antibiotics had outpatient management with hospital discharge within 24 hours. If outpatient management is safe, it could increase convenience and decrease health care use and costs.
Objective: To assess the use and safety of outpatient management of acute appendicitis.
Design, setting, and participants: This cohort study, which is a secondary analysis of the CODA trial, included 776 adults with imaging-confirmed appendicitis who received antibiotics at 25 US hospitals from May 1, 2016, to February 28, 2020.
Exposures: Participants randomized to antibiotics (intravenous then oral) could be discharged from the emergency department based on clinician judgment and prespecified criteria (hemodynamically stable, afebrile, oral intake tolerated, pain controlled, and follow-up confirmed). Outpatient management and hospitalization were defined as discharge within or after 24 hours, respectively.
Main outcomes and measures: Outcomes compared among patients receiving outpatient vs inpatient care included serious adverse events (SAEs), appendectomies, health care encounters, satisfaction, missed workdays at 7 days, and EuroQol 5-dimension (EQ-5D) score at 30 days. In addition, appendectomy incidence among outpatients and inpatients, unadjusted and adjusted for illness severity, was compared.
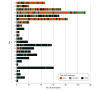
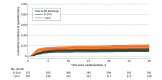
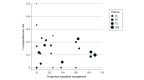
Results: Among 776 antibiotic-randomized participants, 42 (5.4%) underwent appendectomy within 24 hours and 8 (1.0%) did not receive their first antibiotic dose within 24 hours, leaving 726 (93.6%) comprising the study population (median age, 36 years; range, 18-86 years; 462 [63.6%] male; 437 [60.2%] White). Of these participants, 335 (46.1%; site range, 0-89.2%) were discharged within 24 hours, and 391 (53.9%) were discharged after 24 hours. Over 7 days, SAEs occurred in 0.9 (95% CI, 0.2-2.6) per 100 outpatients and 1.3 (95% CI, 0.4-2.9) per 100 inpatients; in the appendicolith subgroup, SAEs occurred in 2.3 (95% CI, 0.3-8.2) per 100 outpatients vs 2.8 (95% CI, 0.6-7.9) per 100 inpatients. During this period, appendectomy occurred in 9.9% (95% CI, 6.9%-13.7%) of outpatients and 14.1% (95% CI, 10.8%-18.0%) of inpatients; adjusted analysis demonstrated a similar difference in incidence (-4.0 percentage points; 95% CI, -8.7 to 0.6). At 30 days, appendectomies occurred in 12.6% (95% CI, 9.1%-16.7%) of outpatients and 19.0% (95% CI, 15.1%-23.4%) of inpatients. Outpatients missed fewer workdays (2.6 days; 95% CI, 2.3-2.9 days) than did inpatients (3.8 days; 95% CI, 3.4-4.3 days) and had similar frequency of return health care visits and high satisfaction and EQ-5D scores.
Conclusions and relevance: These findings support that outpatient antibiotic management is safe for selected adults with acute appendicitis, with no greater risk of complications or appendectomy than hospital care, and should be included in shared decision-making discussions of patient preferences for outcomes associated with nonoperative and operative care.
Trial registration: ClinicalTrials.gov Identifier: NCT02800785.
Conflict of interest statement
Figures
References
-
- Talan DA, Saltzman DJ, Mower WR, et al. ; Olive View–UCLA Appendicitis Study Group . Antibiotics-first versus surgery for appendicitis: a US pilot randomized controlled trial allowing outpatient antibiotic management. Ann Emerg Med. 2017;70(1):1-11.e9. doi: 10.1016/j.annemergmed.2016.08.446 - DOI - PMC - PubMed
-
- American College of Surgeons . COVID-19 guidelines for triage of emergency general surgery patients. Updated December 8, 2020. Accessed November 11, 2021. https://www.facs.org/covid-19/clinical-guidance/elective-case/emergency-...
-
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt). 2010;11(1):79-109. doi: 10.1089/sur.2009.9930 - DOI - PubMed

