Association of BNT162b2 Vaccine Third Dose Receipt With Incidence of SARS-CoV-2 Infection, COVID-19-Related Hospitalization, and Death Among Residents of Long-term Care Facilities, August to October 2021
- PMID: 35796153
- PMCID: PMC9250055
- DOI: 10.1001/jamanetworkopen.2022.19940
Association of BNT162b2 Vaccine Third Dose Receipt With Incidence of SARS-CoV-2 Infection, COVID-19-Related Hospitalization, and Death Among Residents of Long-term Care Facilities, August to October 2021
Abstract
Importance: COVID-19 vaccine might be less immunogenic and effective among residents of long-term care facilities (LTCFs).
Objective: To examine the association of BNT162b2 third dose (first booster dose) with overall SARS-CoV-2 infection, COVID-19 hospitalizations, and mortality among LTCF residents during a nationwide surge of the Delta variant in Israel.
Design, setting, and participants: This observational cohort study conducted nationwide COVID-19 surveillance in LTCFs in Israel between August and October 2021. Participants were residents of LTCFs aged 60 years or older.
Exposures: Vaccination with the third dose of BNT162b2 vaccine vs receipt of 2 doses at least 5 months earlier, based on self-preference and choice.
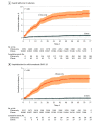
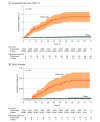
Main outcomes and measures: The cumulative incidences of reverse transcription-polymerase chain reaction (RT-PCR)-confirmed SARS-CoV-2 infection, COVID-19 hospitalizations, and COVID-19-related deaths more than 7 days after vaccination with the third dose were compared between the groups using Kaplan-Meier curves. Hazard ratios (HRs) and 95% CIs were obtained using multivariable Cox regression models.
Results: Among 18 611 residents included in the analysis, 12 715 (68.3%) were female, 463 (2.5%) were from the Arab population, 16 976 (91.2%) were from the general Jewish population, and 618 (3.3%) were from the ultraorthodox Jewish population; the mean (SD) age was 81.1 (9.2) years; 16 082 residents received their first booster dose (third dose) and 2529 were vaccinated with 2 doses at least 5 months earlier. The median (IQR) follow-up durations were 66 (60-70) days among 3-dose recipients and 56 (53-62) days among 2-dose-only recipients; 107 residents had SARS-CoV-2 infection after 7 days following vaccination with the booster dose compared with 185 among the 2-dose only group (cumulative incidence: 0.7% vs 7.5%; adjusted HR, 0.11 [95% CI, 0.07-0.15]). The respective adjusted HRs were 0.07 (95% CI, 0.03-0.14) and 0.10 (95% CI, 0.04-0.24) for the associations of vaccination with the third dose with hospitalization for mild-to-moderate COVID-19 and severe illness. Five COVID-19-related deaths occurred among the third dose vaccinees during the follow-up period compared with 22 among the 2-dose-only vaccinees (cumulative rate: 0.04% vs 0.9%; adjusted HR, 0.04 [95% CI, 0.009-0.16]).
Conclusions and relevance: This cohort study found significant inverse associations between vaccination with the third dose of the BNT162b2 vaccine with overall SARS-CoV-2 infection, COVID-19 hospitalizations, severe disease, and COVID-19-related deaths among LTCF residents during a massive surge caused by the Delta variant in Israel.
Conflict of interest statement
Figures

References
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. . Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819-1829. doi:10.1016/S0140-6736(21)00947-8 - DOI - PMC - PubMed
-
- Haas EJ, McLaughlin JM, Khan F, et al. . Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Infect Dis. 2022;22(3):357-366. doi:10.1016/S1473-3099(21)00566-1 - DOI - PMC - PubMed
-
- Israel Ministry of Health . COVID-19 Dashboard - Coronavirus in Israel, general situation. Published 2021. Accessed September 17, 2021. https://datadashboard.health.gov.il/COVID-19/general
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

