Detecting subclinical anthracycline therapy-related cardiac dysfunction in patients attending Uganda Cancer Institute
- PMID: 35796280
- PMCID: PMC9724070
- DOI: 10.2217/fon-2022-0116
Detecting subclinical anthracycline therapy-related cardiac dysfunction in patients attending Uganda Cancer Institute
Abstract
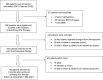
Aims: To investigate the incidence of anthracycline therapy-related cardiac dysfunction (ATRCD) and its predictors among Ugandan cancer patients. Patients & methods: The study recruited 207 cancer patients who were followed for 6 months after ending anthracycline therapy. Global longitudinal strain and troponin-I were the diagnostic tools. Results & conclusions: The cumulative incidences of subclinical and clinical ATRCD were 35.0 and 8.8% respectively. The predictors of clinical ATRCD were HIV infection (hazard ratio [HR]: 3.04; 95% CI: 1.26-7.32; p = 0.013), lower baseline global longitudinal strain (HR: 0.61; 95% CI: 0.53-0.71; p < 0.001) and development of subclinical ATRCD at the end of anthracycline therapy (HR: 6.61; 95% CI: 2.60-16.82; p < 0.001). Cardiac surveillance at baseline and at ending of anthracycline therapy is essential to identify high-risk patients.
Keywords: clinical anthracycline-related cardiac dysfunction; incidence; predictor; subclinical anthracycline therapy-related cardiac dysfunction.
Plain language summary
Anthracyclines are drugs for treating many types of cancers. They may however be harmful to the heart. This anthracycline side effect will first cause subtle heart–cell injury that can be detected and treated if it is handled early. Therefore, this study aims to study patients in the Uganda Cancer Institute to find out how many patients can get and who are likely to get this side effect. We found that 35% of the patients had subtle heart–cell injury and 8.8% had a more severe form of heart–cell injury. The patients who lived with HIV, whose heart was weaker and who got subtle heart–cell injury immediately after treatment were more likely to get the severe form of the side effect. Patients who receive anthracycline therapy need to be monitored closely to prevent serious heart injury.
Figures

References
-
- Middleman E, Luce J, Frei E 3rd. Clinical trials with adriamycin. Cancer 28(4), 844–850 (1971). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
