Lenalidomide, bortezomib and dexamethasone induction therapy for the treatment of newly diagnosed multiple myeloma: a practical review
- PMID: 35796524
- PMCID: PMC9796722
- DOI: 10.1111/bjh.18295
Lenalidomide, bortezomib and dexamethasone induction therapy for the treatment of newly diagnosed multiple myeloma: a practical review
Abstract
For patients with newly diagnosed multiple myeloma, survival outcomes continue to improve significantly: however, nearly all patients will relapse following induction treatment. Optimisation of induction therapy is essential to provide longer term disease control and the current standard of care for most patients incorporates an immunomodulatory agent and proteasome inhibitor, most commonly lenalidomide and bortezomib in combination with dexamethasone (RVD), with maintenance until progression. Historically there has been limited access to RVD as an induction strategy outside of the United States; fortunately, there is now increasing access worldwide. This review discusses the rationale for use of RVD as induction therapy and aims to provide guidance in prescribing this regimen in order to optimise efficacy while minimising the toxicities of treatment. We also highlight the increasing evidence for the utility of addition of a monoclonal antibody to the RVD backbone to deepen responses and potentially provide longer disease control.
© 2022 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
Georgia J. McCaughan reports advisory board participation and honoraria from Janssen. Paul G. Richardson reports institutional grant support from Bristol Myers Squibb/Celgene, Karyopharm, Oncopeptides AB, and Takeda; consulting fees for advisory committee service from Bristol Myers Squibb/Celgene, Karyopharm, Oncopeptides AB, GlaxoSmithKline, Janssen, Sanofi, and Takeda. Sara Gandolfi and John J. Moore have no conflicts of interest.
Figures
References
-
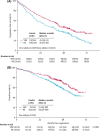
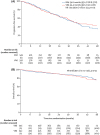
- Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem‐cell transplant (SWOG S0777): a randomised, open‐label, phase 3 trial. Lancet. 2017;389(10068):519–27. - PMC - PubMed
-
- Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

