Lessons learned: the first consecutive 1000 patients of the CCCMunichLMU Molecular Tumor Board
- PMID: 35796778
- PMCID: PMC9261163
- DOI: 10.1007/s00432-022-04165-0
Lessons learned: the first consecutive 1000 patients of the CCCMunichLMU Molecular Tumor Board
Abstract
Purpose: In 2016, the University of Munich Molecular Tumor Board (MTB) was implemented to initiate a precision oncology program. This review of cases was conducted to assess clinical implications and functionality of the program, to identify current limitations and to inform future directions of these efforts.
Methods: Charts, molecular profiles, and tumor board decisions of the first 1000 consecutive cases (01/2016-03/2020) were reviewed. Descriptive statistics were applied to describe relevant findings.
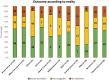
Results: Of the first 1000 patients presented to the MTB; 914 patients received comprehensive genomic profiling. Median age of patients was 56 years and 58% were female. The most prevalent diagnoses were breast (16%) and colorectal cancer (10%). Different types of targeted or genome-wide sequencing assays were used; most of them offered by the local department of pathology. Testing was technically successful in 88%. In 41% of cases, a genomic alteration triggered a therapeutic recommendation. The fraction of patients receiving a tumor board recommendation differed significantly between malignancies ranging from over 50% in breast or biliary tract to less than 30% in pancreatic cancers. Based on a retrospective chart review, 17% of patients with an MTB recommendation received appropriate treatment.
Conclusion: Based on these retrospective analyses, patients with certain malignancies (breast and biliary tract cancer) tend to be more likely to have actionable variants. The low rate of therapeutic implementation (17% of patients receiving a tumor board recommendation) underscores the importance of meticulous follow-up for these patients and ensuring broad access to innovative therapies for patients receiving molecular tumor profiling.
Keywords: Comprehensive genomic profiling; Molecular tumor board; Personalized medicine; Precision oncology; Targeted therapy.
© 2022. The Author(s).
Conflict of interest statement
Kathrin Heinrich: Honoraria: Roche, Consulting or Advisory Role: Servier, Travel, Accommodations, Expenses: Lilly, AMGEN, Celgene. Lisa Miller-Phillips: reimbursement for travel by Merck. Michael von Bergwelt-Baildon: honoraria, research funding and speakers bureau: MSD Sharp & Dohme, Novartis, Roche, KITE/Gilead, Bristol-Myers Squibb, Astellas, Mologen, Miltenyi. Julian Holch: Advisory Role: Roche, Honoraria: Roche, Travel Support: Novartis. Philipp A. Greif: Astra Zeneca: Medical Advisory Board 2020. Rachel Würstlein: Served as advisor, consultant, speaker and travel grant: Agendia, Amgen, Aristo, Astra Zeneca, Boeringer Ingelheim, Carl Zeiss, Celgene, Clinsol, Daiichi-Sankyo, Eisai, ExactSciences, Genomic Health, Gilead, Glaxo Smith Kline, Hexal, Lilly, Medstrom Medical, MSC, Mundipharma, Nanostrin, Novartis, Odonate. Christine Spitzweg: Honoraria for lectures or advisory board: Lilly, Roche, Eisai, Ipsen, Bayer, Blueprint Medicine. Max Seidensticker: grants and personal fees from Sirtex, Bayer Healthcare, personal fees from Siemens, Cook, Boston Scientific, Astra Zeneca. Stefanie Corradini: honoraria or travel/accommodation expenses: Elekta, Brainlab, Viewray, C-RAD, Roche, research funding: Elekta, Brainlab, Viewray. Andreas Jung: Consulting or advisory role: Amgen, AstraZeneca, BIocartis, Novartis; Speakers’ Bureau: Amgen, AstraZeneca, Bayer Pharmaceuticals, Biocartis, BMS, Boehringer Ingelheim, Merck KgA, Lilly, MSD, Novartis, Qiagen, QuIP GmbH, Roche Pharma, Takeda, Thermo Fisher; Travel/Accommodation/Expenses: Amgen, AstraZeneca, Bayer Pharmaceuticals, Biocartis, BMS, Boehringer Ingelheim, Merck KgA, Lilly, MSD, Novartis, Qiagen, QuIP GmbH, Roche Pharma, Takeda, Thermo Fisher. Jörg Kumbrink: received honoraria and reimbursement for travel and accommodation for participants in advisory boards from AstraZeneca, Novartis, and Roche Pharma. Thomas Kirchner: Consulting/Advisory: Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Merck KGaA, MSD, Novartis, Pfizer, Qiagen, Roche, Takeda; Research Funding: Merck, Roche; Speaker: Merck, Astra Zeneca. Frederick Klauschen: Co-Founder AI-BIH/Charité-Spinoff Aignostics GmbH; Advisor BMS, Novartis, Roche, Lilly, Agilent, AstraZeneca and Merck. Klaus Metzeler: Beratertätigkeit: Celgene/BMS, Novartis, Jazz Pharmaceuticals, Pfizer; Honorare: Celgene/BMS, Daiichi Sankyo, Astellas, AbbVie, Novartis, Janssen; Finanzierung wissenschaftlicher Untersuchungen: Celgene. Volker Heinemann: Honoraria: Merck, Roche, Celgene, AMGEN, Sanofi, Lilly, SIRTEX, Boehringer-Ingelheim, Taiho, Servier; Consulting or Advisory Board: Merck, Roche, AMGEN, Sanofi, SIRTEX, Servier, Celgene, Boehringer-Ingelheim, Halozyme, MSD, BMS; Research funding: MERCK, Roche, AMGEN, SIRTEX, Servier, Celgene, Boehringer-Ingelheim, Shire; Travel Accommodation expenses: MERCK, Roche, AMGEN, SIRTEX, Servier, Shire, MSD, BMS. Christoph Benedikt Westphalen: has received honoraria from Bayer, Celgene, Ipsen, F. Hoffmann-La Roche Ltd, Servier, and Taiho, served in a consulting/advisory role for BMS, Celgene, Merck, Shire/Baxalta, Rafael Pharmaceuticals, RedHill BioPharma, and F. Hoffmann-La Roche Ltd, has received travel/accommodation expenses from Bayer, Celgene, RedHill BioPharma, F. Hoffmann-La Roche Ltd, Servier, and Taiho and has received research funding from F. Hoffmann-La Roche Ltd.. Frank Ziemann, Korbinian Hasselmann, Katharina Rühlmann, Madeleine Flach, Dorottya Biro, Tobias Herold, Louisa von Baumgarten, Irmela Jeremias, Bernhard Renz, Philipp Baumeister, Elisabetta Goni: none declared. Jozefina Casuscelli, Amanda Tufman: no information.
Figures
References
-
- Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson D, Murphy AG, Oh DY, Dotan E, Catenacci DV, Van Cutsem E, Ji T, Lihou CF, Zhen H, Feliz L, Vogel A (2020) Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol 21(5):671–684 - PMC - PubMed
-
- Bekaii-Saab TS, Valle JW, Cutsem EV, Rimassa L, Furuse J, Ioka T, Melisi D, Macarulla T, Bridgewater J, Wasan H, Borad MJ, Abou-Alfa GK, Jiang P, Lihou CF, Zhen H, Asatiani E, Feliz L, Vogel A (2020) FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol 16:2385–2399 - PMC - PubMed
-
- Bernhardt EB, Chamberlin MD, Gorlov IP, de Abreu FB, Bloch KJ, Peterson JD, Tsongalis GJ, Shirai K, Dragnev KH, Miller TW, Tafe LJ (2020) Molecular matching and treatment strategies for advanced stage lung cancer at Dartmouth-Hitchcock Medical Center: a three-year review of a Molecular Tumor Board. Pract Lab Med 21:e00174 - PMC - PubMed
-
- Bitzer M, Ostermann L, Horger M, Biskup S, Schulze M, Ruhm K, Hilke F, Oner O, Nikolaou K, Schroeder C, Riess O, Fend F, Zips D, Hinterleitner M, Zender L, Tabatabai G, Beha J, Malek NP (2020) Next-generation sequencing of advanced GI tumors reveals individual treatment options. JCO Precis Oncol 4:258–271 - PMC - PubMed
-
- Brock A, Huang S (2017) Precision oncology: between vaguely right and precisely wrong. Cancer Res 77(23):6473–6479 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical