Interaction of immune checkpoint PD-1 and chemokine receptor 4 (CXCR4) promotes a malignant phenotype in pancreatic cancer cells
- PMID: 35797269
- PMCID: PMC9262213
- DOI: 10.1371/journal.pone.0270832
Interaction of immune checkpoint PD-1 and chemokine receptor 4 (CXCR4) promotes a malignant phenotype in pancreatic cancer cells
Abstract
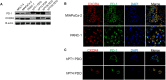
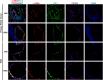
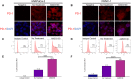
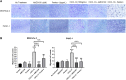
Despite recent therapeutic advances, pancreatic ductal adenocarcinoma (PDAC) remains a devastating disease with limited therapeutic options. Immune checkpoint inhibitors (ICIs) have demonstrated promising results in many cancers, but thus far have yielded little clinical benefit in PDAC. Based on recent combined targeting of programmed cell death protein-1 (PD-1) and C-X-C chemokine receptor 4 (CXCR4) in patient-derived xenografts (PDXs) and a pilot clinical trial, we sought to elucidate potential interactions between PD-1 and CXCR4. We observed concomitant expression and direct interaction of PD-1 and CXCR4 in PDAC cells. This interaction was disrupted upon CXCR4 antagonism with AMD3100 and led to increased cell surface expression of PD-1. Importantly, CXCR4-mediated PDAC cell migration was also blocked by PD-1 inhibition. Our work provides a possible mechanism by which prior studies have demonstrated that combined CXCR4 and PD-1 inhibition leads to decreased tumor growth. This is the first report investigating PD-1 and CXCR4 interactions in PDAC cells and our results can serve as the basis for further investigation of combined therapeutic targeting of CXCR4 and PD-1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
CXCR4-CXCL12-CXCR7 and PD-1/PD-L1 in Pancreatic Cancer: CXCL12 Predicts Survival of Radically Resected Patients.Cells. 2022 Oct 22;11(21):3340. doi: 10.3390/cells11213340. Cells. 2022. PMID: 36359736 Free PMC article.
-
Immune Checkpoint Inhibition for Pancreatic Ductal Adenocarcinoma: Current Limitations and Future Options.Front Immunol. 2018 Aug 15;9:1878. doi: 10.3389/fimmu.2018.01878. eCollection 2018. Front Immunol. 2018. PMID: 30158932 Free PMC article. Review.
-
Expression and role of the immune checkpoint regulator PD-L1 in the tumor-stroma interplay of pancreatic ductal adenocarcinoma.Front Immunol. 2023 Jun 28;14:1157397. doi: 10.3389/fimmu.2023.1157397. eCollection 2023. Front Immunol. 2023. PMID: 37449210 Free PMC article.
-
Dual Stromal Targeting Sensitizes Pancreatic Adenocarcinoma for Anti-Programmed Cell Death Protein 1 Therapy.Gastroenterology. 2022 Nov;163(5):1267-1280.e7. doi: 10.1053/j.gastro.2022.06.027. Epub 2022 Jun 17. Gastroenterology. 2022. PMID: 35718227 Free PMC article.
-
Clinicopathological significance and prognostic role of chemokine receptor CXCR4 expression in pancreatic ductal adenocarcinoma, a meta-analysis and literature review.Int J Surg. 2019 May;65:32-38. doi: 10.1016/j.ijsu.2019.03.009. Epub 2019 Mar 19. Int J Surg. 2019. PMID: 30902754 Review.
Cited by
-
Analysis of C-X-C motif chemokine receptors in breast cancer: potential value in immunotherapy and prognostic prediction.Ann Transl Med. 2022 Dec;10(24):1379. doi: 10.21037/atm-22-6056. Ann Transl Med. 2022. PMID: 36660642 Free PMC article.
-
The CXCR4 antagonist R54 targets epithelial-mesenchymal transition (EMT) in human ovarian cancer cells.PLoS One. 2024 Dec 19;19(12):e0314735. doi: 10.1371/journal.pone.0314735. eCollection 2024. PLoS One. 2024. PMID: 39700131 Free PMC article.
-
Unraveling the mechanisms of irAEs in endometrial cancer immunotherapy: insights from FAERS and scRNA-seq data.Sci Rep. 2025 May 28;15(1):18645. doi: 10.1038/s41598-025-02723-3. Sci Rep. 2025. PMID: 40436981 Free PMC article.
-
Define cancer-associated fibroblasts (CAFs) in the tumor microenvironment: new opportunities in cancer immunotherapy and advances in clinical trials.Mol Cancer. 2023 Oct 2;22(1):159. doi: 10.1186/s12943-023-01860-5. Mol Cancer. 2023. PMID: 37784082 Free PMC article. Review.
-
PD-1 interactome in osteosarcoma: identification of a novel PD-1/AXL interaction conserved between humans and dogs.Cell Commun Signal. 2024 Dec 18;22(1):605. doi: 10.1186/s12964-024-01935-w. Cell Commun Signal. 2024. PMID: 39696578 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

