Formylpeptide receptor 2: Nomenclature, structure, signalling and translational perspectives: IUPHAR review 35
- PMID: 35797341
- PMCID: PMC9545948
- DOI: 10.1111/bph.15919
Formylpeptide receptor 2: Nomenclature, structure, signalling and translational perspectives: IUPHAR review 35
Abstract
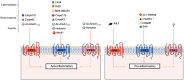
We discuss the fascinating pharmacology of formylpeptide receptor 2 (FPR2; often referred to as FPR2/ALX since it binds lipoxin A4 ). Initially identified as a low-affinity 'relative' of FPR1, FPR2 presents complex and diverse biology. For instance, it is activated by several classes of agonists (from peptides to proteins and lipid mediators) and displays diverse expression patterns on myeloid cells as well as epithelial cells and endothelial cells, to name a few. Over the last decade, the pharmacology of FPR2 has progressed from being considered a weak chemotactic receptor to a master-regulator of the resolution of inflammation, the second phase of the acute inflammatory response. We propose that exploitation of the biology of FPR2 offers innovative ways to rectify chronic inflammatory states and represents a viable avenue to develop novel therapies. Recent elucidation of FPR2 structure will facilitate development of the anti-inflammatory and pro-resolving drugs of next decade.
Keywords: ALXR; FPR; N-formylated peptides; annexin-A1; lipoxin A4; resolution of inflammation.
© 2022 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
MP has conducted commercial projects with Bristol Myers Squibb, Palatin Technologies and SynAct Pharma AS. He is on the Scientific Advisory Board of ResoTher Pharma AS, which is developing ANXA1‐derived peptides for cardiovascular settings. He consults for Bristol Myers Squibb, SynAct Pharma and TXP Pharma. CXQ consults for Shandong Hanfang Pharmaceutical Co. Ltd. All other authors have nothing to declare.
Figures

References
-
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐Hosaini, K. , Back, M. , Barnes, N. M. , Bathgate, R. , … Ye, R. D. (2021). The concise guide to pharmacology 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(Suppl 1), S27–S156. - PubMed
-
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Boison, D. , Burns, K. E. , Dessauer, C. , Gertsch, J. , Helsby, N. A. , Izzo, A. A. , Koesling, D. , … Wong, S. S. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Enzymes. British Journal of Pharmacology, 178(S1), S313–S411. 10.1111/bph.15542 - DOI - PubMed
-
- Ammendola, R. , Russo, L. , De Felice, C. , Esposito, F. , Russo, T. , & Cimino, F. (2004). Low‐affinity receptor‐mediated induction of superoxide by N‐formyl‐methionyl‐leucyl‐phenylalanine and WKYMVm in IMR90 human fibroblasts. Free Radical Biology & Medicine, 36, 189–200. - PubMed
-
- Andrews, D. , & Godson, C. (2021). Lipoxins and synthetic lipoxin mimetics: Therapeutic potential in renal diseases. Biochimica et Biophysica Acta ‐ Molecular and Cell Biology of Lipids, 1866, 158940. - PubMed
-
- Arita, M. , Ohira, T. , Sun, Y. P. , Elangovan, S. , Chiang, N. , & Serhan, C. N. (2007). Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. Journal of Immunology, 178, 3912–3917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

