Structured sampling of olfactory input by the fly mushroom body
- PMID: 35797998
- PMCID: PMC9413950
- DOI: 10.1016/j.cub.2022.06.031
Structured sampling of olfactory input by the fly mushroom body
Abstract
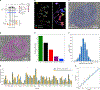
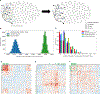
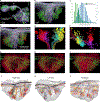
Associative memory formation and recall in the fruit fly Drosophila melanogaster is subserved by the mushroom body (MB). Upon arrival in the MB, sensory information undergoes a profound transformation from broadly tuned and stereotyped odorant responses in the olfactory projection neuron (PN) layer to narrowly tuned and nonstereotyped responses in the Kenyon cells (KCs). Theory and experiment suggest that this transformation is implemented by random connectivity between KCs and PNs. However, this hypothesis has been challenging to test, given the difficulty of mapping synaptic connections between large numbers of brain-spanning neurons. Here, we used a recent whole-brain electron microscopy volume of the adult fruit fly to map PN-to-KC connectivity at synaptic resolution. The PN-KC connectome revealed unexpected structure, with preponderantly food-responsive PN types converging at above-chance levels on downstream KCs. Axons of the overconvergent PN types tended to arborize near one another in the MB main calyx, making local KC dendrites more likely to receive input from those types. Overconvergent PN types preferentially co-arborize and connect with dendrites of αβ and α'β' KC subtypes. Computational simulation of the observed network showed degraded discrimination performance compared with a random network, except when all signal flowed through the overconvergent, primarily food-responsive PN types. Additional theory and experiment will be needed to fully characterize the impact of the observed non-random network structure on associative memory formation and recall.
Keywords: Drosophila melanogaster; Kenyon cell; connectomics; electron microscopy; memory; mushroom body; neural circuit; neuroanatomy; olfaction; projection neuron.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

