Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant
- PMID: 35798000
- PMCID: PMC9212999
- DOI: 10.1016/j.xcrm.2022.100679
Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant
Abstract
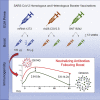
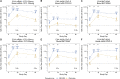
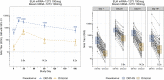
The Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exhibits reduced susceptibility to vaccine-induced neutralizing antibodies, requiring a boost to generate protective immunity. We assess the magnitude and short-term durability of neutralizing antibodies after homologous and heterologous boosting with mRNA and Ad26.COV2.S vaccines. All prime-boost combinations substantially increase the neutralization titers to Omicron, although the boosted titers decline rapidly within 2 months from the peak response compared with boosted titers against the prototypic D614G variant. Boosted Omicron neutralization titers are substantially higher for homologous mRNA vaccine boosting, and for heterologous mRNA and Ad26.COV2.S vaccine boosting, compared with homologous Ad26.COV2.S boosting. Homologous mRNA vaccine boosting generates nearly equivalent neutralizing activity against Omicron sublineages BA.1, BA.2, and BA.3 but modestly reduced neutralizing activity against BA.2.12.1 and BA.4/BA.5 compared with BA.1. These results have implications for boosting requirements to protect against Omicron and future variants of SARS-CoV-2. This trial was conducted under ClincalTrials.gov: NCT04889209.
Keywords: BA.2.12.1; BA.4/BA.5; COVID-19; Omicron variant; SARS-CoV-2; booster; mRNA vaccine; neutralizing antibody; recombinant adenovirus vaccine; sublineage.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.L.A., C.D.I., C.M.P., D.S., R.P.C., M.E.D., A.E., H.M.E.S., R.E.R., M.B., A.C.K., T.M.B., D.D., R.N.C., J.I.A., S.C., J.A.Z., S.U.N., E.R.B., and D.J.P. report no competing interests. K.E.L. receives grant awards from Pfizer, Inc., COVID-19 vaccine research. L.A.J.’s institution receives grant funding from NIH and CDC for vaccine-related assessments, including those of COVID-19 vaccines. A.R.B. has grant funding from Pfizer, Janssen, Merck, and Cyanvac for non-COVID-19-related work and serves as a consultant for GSK and Janssen. C.A.R.'s institution has received funds to conduct clinical research from the National Institutes of Health, CDC, BioFire, Inc., Genentech, GSK, Janssen, MedImmune, Merck, Micron, Moderna, Novavax, PaxVax, Pfizer, Regeneron, and Sanofi-Pasteur. She is co-inventor of patented RSV vaccine technology, which has been licensed to Meissa Vaccines, Inc. J.M.M. has served as a consultant for Merck, Sharp, and Dohme for non-Covid-related work. C.J. receives funding from the Bill and Melinda Gates Foundation, NIH, and CDC, consults for Gilead and Abbvie, serves on a DSMB for MedPace, and receives royalties from UpToDate. M.J.M. has laboratory research and clinical trials contracts for vaccines or MAB versus SARS-CoV-2 with Lilly, Pfizer (exclusive of the current work), and Sanofi and personal fees for Scientific Advisory Board service from Merck, Meissa Vaccines, Inc., and Pfizer. R.C.B. receives funding for vaccine trials from Path Nipah and Pfizer. R.W.F. receives funding to perform clinical trials from Pfizer, Moderna, Astra Zeneca, and Emergent Health, and he serves on advisory boards for Johnson & Johnson, Merck, Sanofi Pasteur, and Seqirus. S.E. receives funding to her institution from Sanofi Pasteur for a non-COVID-19 vaccine study. K.M.N. holds a grant from Pfizer, without salary support, for a COVID-19 vaccine study and salary support from the National Institutes of Health (NIH) for work on multiple COVID-19 vaccine trials. D.S.S. is supported by grant awards from NIH/NIAID. P.C.R. and J.H.B. report a pending US patent application no. 63/025918 entitled “Coronavirus RNA vaccines and methods of use.” D.C.M. receives funding from NIH and Moderna for laboratory studies of COVID-19 vaccine antibody responses. M.S.S. receives funding from Moderna, Inc., and Ocugen. D.C.M. receives funding from Moderna, Inc.
Figures


References
-
- CDC . US Department of Health and Human Services, CDC; 2022. Science Brief: Omicron (B.1.1.529) Variant.https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scienti...
-
- CDC . US Department of Health and Human Services; 2022. Potential Rapid Increase of Omicron Variant Infection in the United States.https://www.cdc.gov/coronavirus/2019-ncov/science/forecasting/mathematic...
-
- Edara V.V., Manning K.E., Ellis M., Lai L., Moore K.M., Foster S.L., Floyd K., Davis-Gardner M.E., Mantus G., Nyhoff L.E., et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep. Med. 2022;3:100529. doi: 10.1016/j.xcrm.2022.100529. - DOI - PMC - PubMed
-
- Houriiyah T., Moir M., Everatt J., Giovanetti M., Scheepers C., Wilkinson E., Subramoney K., Moyo S., Amoako D.G., Baxter C., et al. Continued Emergence and Evolution of Omicron in South Africa: New BA.4 and BA.5 Lineages. medRxiv. 2022 doi: 10.1101/2022.05.01.22274406. Preprint at. - DOI
-
- Ariën K.K., Heyndrickx L., Michiels J., Vereecken K., Van Lent K., Coppens S., Willems B., Pannus P., Martens G.A., Van Esbroeck M., et al. Three doses of BNT162b2 vaccine confer neutralising antibody capacity against the SARS-CoV-2 Omicron variant. NPJ Vaccines. 2022;7:35. doi: 10.1038/s41541-022-00459-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
- UM1 AI148372/AI/NIAID NIH HHS/United States
- 75N93021C00017/AI/NIAID NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- UM1 AI148452/AI/NIAID NIH HHS/United States
- UM1 AI148573/AI/NIAID NIH HHS/United States
- UM1 AI148373/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- U54 CA260563/CA/NCI NIH HHS/United States
- HHSN272201400004C/AI/NIAID NIH HHS/United States
- 75N93019C00050/AI/NIAID NIH HHS/United States
- UM1 AI148574/AI/NIAID NIH HHS/United States
- UM1 AI148689/AI/NIAID NIH HHS/United States
- UM1 AI148450/AI/NIAID NIH HHS/United States
- T32 AI007524/AI/NIAID NIH HHS/United States
- UM1 AI148575/AI/NIAID NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

