Factors Associated with Delayed or Missed Second-Dose mRNA COVID-19 Vaccination among Persons >12 Years of Age, United States
- PMID: 35798008
- PMCID: PMC9328898
- DOI: 10.3201/eid2808.220557
Factors Associated with Delayed or Missed Second-Dose mRNA COVID-19 Vaccination among Persons >12 Years of Age, United States
Abstract
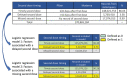
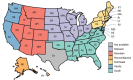
To identify demographic factors associated with delaying or not receiving a second dose of the 2-dose primary mRNA COVID-19 vaccine series, we matched 323 million single Pfizer-BioNTech (https://www.pfizer.com) and Moderna (https://www.modernatx.com) COVID-19 vaccine administration records from 2021 and determined whether second doses were delayed or missed. We used 2 sets of logistic regression models to examine associated factors. Overall, 87.3% of recipients received a timely second dose (≤42 days between first and second dose), 3.4% received a delayed second dose (>42 days between first and second dose), and 9.4% missed the second dose. Persons more likely to have delayed or missed the second dose belonged to several racial/ethnic minority groups, were 18-39 years of age, lived in more socially vulnerable areas, and lived in regions other than the northeastern United States. Logistic regression models identified specific subgroups for providing outreach and encouragement to receive subsequent doses on time.
Keywords: 2019 novel coronavirus disease; 2019-nCoV vaccine mRNA; COVID-19; Moderna COVID-19 vaccine; SARS-CoV-2; coronavirus disease; mRNA vaccine; respiratory infections; severe acute respiratory syndrome coronavirus 2; vaccination; vaccine delay; vaccine timing; vaccines; viruses; zoonoses.
Figures
References
-
- Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922–4. 10.15585/mmwr.mm6950e2 - DOI - PMC - PubMed
-
- Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653–6.Error! Hyperlink reference not valid. 10.15585/mmwr.mm695152e1 - DOI - PMC - PubMed
-
- Dooling K, Marin M, Wallace M, McClung N, Chamberland M, Lee GM, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 vaccine–UnitedStates, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657–60. 10.15585/mmwr.mm695152e2 - DOI - PMC - PubMed
-
- Kriss JL, Reynolds LE, Wang A, Stokley S, Cole MM, Harris LQ, et al. ; CDC COVID-19 Vaccine Task Force. COVID-19 vaccine second-dose completion and interval between first and second doses among vaccinated persons—United States, December 14, 2020–February 14, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:389–95. 10.15585/mmwr.mm7011e2 - DOI - PMC - PubMed
-
- Wallace M, Woodworth KR, Gargano JW, Scobie HM, Blain AE, Moulia D, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12–15 years–United States, May 2021. MMWR Morb Mortal Wkly Rep. 2021;70:749–52. 10.15585/mmwr.mm7020e1 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

