Tumor Cells Modulate Macrophage Phenotype in a Novel In Vitro Co-Culture Model of the NSCLC Tumor Microenvironment
- PMID: 35798240
- PMCID: PMC9529910
- DOI: 10.1016/j.jtho.2022.06.011
Tumor Cells Modulate Macrophage Phenotype in a Novel In Vitro Co-Culture Model of the NSCLC Tumor Microenvironment
Abstract
Introduction: Macrophage phenotype in the tumor microenvironment correlates with prognosis in NSCLC. Immunosuppressive macrophages promote tumor progression, whereas proinflammatory macrophages may drive an antitumor immune response. How individual NSCLCs affect macrophage phenotype is a major knowledge gap.
Methods: To systematically study the impact of lung cancer cells on macrophage phenotypes, we developed an in vitro co-culture model that consisted of molecularly and clinically annotated patient-derived NSCLC lines, human cancer-associated fibroblasts, and murine macrophages. Induced macrophage phenotype was studied through quantitative real-time polymerase chain reaction and validated in vivo using NSCLC xenografts through quantitative immunohistochemistry and clinically with The Cancer Genome Atlas (TCGA)-"matched" patient tumors.
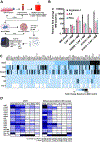
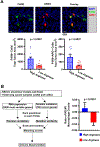
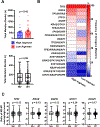
Results: A total of 72 NSCLC cell lines were studied. The most frequent highly induced macrophage-related gene was Arginase-1, reflecting an immunosuppressive M2-like phenotype. This was independent of multiple clinicopathologic factors, which also did not affect M2:M1 ratios in matched TCGA samples. In vivo, xenograft tumors established from high Arginase-1-inducing lines (Arghi) had a significantly elevated density of Arg1+ macrophages. Matched TCGA clinical samples to Arghi NSCLC lines had a significantly higher ratio of M2:M1 macrophages (p = 0.0361).
Conclusions: In our in vitro co-culture model, a large panel of patient-derived NSCLC lines most frequently induced high-expression Arginase-1 in co-cultured mouse macrophages, independent of major clinicopathologic and oncogenotype-related factors. Arghi cluster-matched TCGA tumors contained a higher ratio of M2:M1 macrophages. Thus, this in vitro model reproducibly characterizes how individual NSCLC modulates macrophage phenotype, correlates with macrophage polarization in clinical samples, and can serve as an accessible platform for further investigation of macrophage-specific therapeutic strategies.
Keywords: In vitro co-culture model; Macrophage phenotype; Non–small cell lung cancer; Tumor microenvironment.
Copyright © 2022 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Ruiz-Cordero R, Devine WP. Targeted Therapy and Checkpoint Immunotherapy in Lung Cancer. Surg Pathol Clin 2020;13:17–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

