Plasma cell but not CD20-mediated B-cell depletion protects from bleomycin-induced lung fibrosis
- PMID: 35798357
- PMCID: PMC9684624
- DOI: 10.1183/13993003.01469-2021
Plasma cell but not CD20-mediated B-cell depletion protects from bleomycin-induced lung fibrosis
Abstract
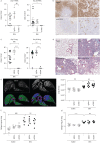
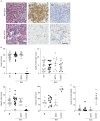
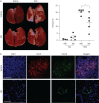
Idiopathic pulmonary fibrosis (IPF) is an interstitial lung disease associated with chronic inflammation and tissue remodelling leading to fibrosis, reduced pulmonary function, respiratory failure and death. Bleomycin (Blm)-induced lung fibrosis in mice replicates several clinical features of human IPF, including prominent lymphoid aggregates of predominantly B-cells that accumulate in the lung adjacent to areas of active fibrosis. We have shown previously a requirement for B-cells in the development of Blm-induced lung fibrosis in mice. To determine the therapeutic potential of inhibiting B-cell function in pulmonary fibrosis, we examined the effects of anti-CD20 B-cell ablation therapy to selectively remove mature B-cells from the immune system and inhibit Blm-induced lung fibrosis. Anti-CD20 B-cell ablation did not reduce fibrosis in this model; however, immune phenotyping of peripheral blood and lung resident cells revealed that anti-CD20-treated mice retained a high frequency of CD19+ CD138+ plasma cells. Interestingly, high levels of CD138+ cells were also identified in the lung tissue of patients with IPF, consistent with the mouse model. Treatment of mice with bortezomib, which depletes plasma cells, reduced the level of Blm-induced lung fibrosis, implicating plasma cells as important effector cells in the development and progression of pulmonary fibrosis.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: S.E. Mutsaers, C.M. Prêle, R.J. McAnulty, G.J. Laurent, G. Hoyne, D.A. Knight, R.J. O'Donoghue and M. Ernst received grants from National Health and Medical Research Council (NHMRC), project grants ID APP1067511. R.J. McAnulty, S.E. Mutsaers, C.M. Prêle and D.R. Pearce received grants from British Lung Foundation, project grant number PPRG15-10. T. Miles reports that Genentech provided the anti-CD20 antibody, and received UWA Research Training Program Scholarship and the Lung Foundation Australia Bill van Nierop PhD Scholarship. All other authors have nothing to disclose.
Figures



Comment in
-
Plasma cells: a feasible therapeutic target in pulmonary fibrosis?Eur Respir J. 2022 Nov 24;60(5):2201748. doi: 10.1183/13993003.01748-2022. Print 2022 Nov. Eur Respir J. 2022. PMID: 36423920 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
