Case of reactive sacroiliitis possibly induced by an mRNA coronavirus disease vaccine
- PMID: 35798496
- PMCID: PMC9263939
- DOI: 10.1136/bcr-2022-249063
Case of reactive sacroiliitis possibly induced by an mRNA coronavirus disease vaccine
Abstract
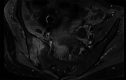
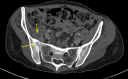
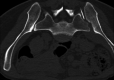
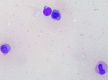
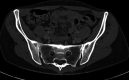
A woman in her 30s received a second dose, first booster, Corminaty vaccine against the SARS-CoV-2. Three days later, the patient developed unilateral sacroiliitis. A pelvic scan revealed inflammatory joint edges, bone erosion and a heterogeneous mass of 2.5 cm in the psoas muscle. Joint puncture revealed no microcrystalline deposits, but bone marrow cells, erythroblast were identified. The standard bacterial cultures and culture for mycobacteria were negative. HLA B27 was negative, and no seroconversion was identified for HIV, Epstein-Barr virus, cytomegalovirus, chlamydia or Quantiferon. Two months later, the sacroiliitis resolved.The aetiologic approach of this erosive unilateral acute sacroiliitis in a person naïve to rheumatologic pathology was negative for inflammatory or infectious sacroiliitis. Arthralgias after vaccination are expected. Arthritis is less common, and acute sacroiliitis has not yet been described. Acute sacroiliitis may be considered a reactive sacroiliitis to the anti-COVID-19 mRNA vaccine.
Keywords: Anklosing spondylitis; COVID-19; Healthcare improvement and patient safety; Immunological products and vaccines; Vaccination/immunisation.
© BMJ Publishing Group Limited 2022. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
