Motor Neuron Disease Systematic Multi-Arm Adaptive Randomised Trial (MND-SMART): a multi-arm, multi-stage, adaptive, platform, phase III randomised, double-blind, placebo-controlled trial of repurposed drugs in motor neuron disease
- PMID: 35798516
- PMCID: PMC9263927
- DOI: 10.1136/bmjopen-2022-064173
Motor Neuron Disease Systematic Multi-Arm Adaptive Randomised Trial (MND-SMART): a multi-arm, multi-stage, adaptive, platform, phase III randomised, double-blind, placebo-controlled trial of repurposed drugs in motor neuron disease
Abstract
Introduction: Motor neuron disease (MND) is a rapidly fatal neurodegenerative disease. Despite decades of research and clinical trials there remains no cure and only one globally approved drug, riluzole, which prolongs survival by 2-3 months. Recent improved mechanistic understanding of MND heralds a new translational era with many potential targets being identified that are ripe for clinical trials. Motor Neuron Disease Systematic Multi-Arm Adaptive Randomised Trial (MND-SMART) aims to evaluate the efficacy of drugs efficiently and definitively in a multi-arm, multi-stage, adaptive trial. The first two drugs selected for evaluation in MND-SMART are trazodone and memantine.
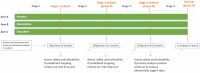
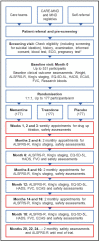
Methods and analysis: Initially, up to 531 participants (177/arm) will be randomised 1:1:1 to oral liquid trazodone, memantine and placebo. The coprimary outcome measures are the Amyotrophic Lateral Sclerosis Functional Rating Scale Revised (ALSFRS-R) and survival. Comparisons will be conducted in four stages. The decision to continue randomising to arms after each stage will be made by the Trial Steering Committee who receive recommendations from the Independent Data Monitoring Committee. The primary analysis of ALSFRS-R will be conducted when 150 participants/arm, excluding long survivors, have completed 18 months of treatment; if positive the survival effect will be inferentially analysed when 113 deaths have been observed in the placebo group. The trial design ensures that other promising drugs can be added for evaluation in planned trial adaptations. Using this novel trial design reduces time, cost and number of participants required to definitively (phase III) evaluate drugs and reduces exposure of participants to potentially ineffective treatments.
Ethics and dissemination: MND-SMART was approved by the West of Scotland Research Ethics Committee on 2 October 2019. (REC reference: 19/WS/0123) Results of the study will be submitted for publication in a peer-reviewed journal and a summary provided to participants.
Trial registration numbers: European Clinical Trials Registry (2019-000099-41); NCT04302870.
Keywords: Clinical trials; Motor neurone disease; Neurology; STATISTICS & RESEARCH METHODS.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: CJW is a member of the independent data monitoring committee for a trial of masitinib in ALS, for which his institution receives funding from AB Science. In the last 3 years, JC has received support from the Efficacy and Evaluation (EME) Programme, a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership and the Health Technology Assessment (HTA) Programme (NIHR), the UK MS Society, the US National MS Society and the Rosetrees Trust. He is supported in part by the NIHR University College London Hospitals (UCLH) Biomedical Research Centre, London, UK. He has been a local principal investigator for a trial in MS funded by the Canadian MS society. A local principal investigator for commercial trials funded by: Actelion, Novartis and Roche; and has taken part in advisory boards/consultancy for Azadyne, Janssen, Merck, NervGen, Novartis and Roche.
Figures
References
-
- Miller RG, Mitchell JD, Lyon M. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 2002;2:CD001447. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
