Estimating the Risk for Secondary Cancer After Targeted α-Therapy with 211At Intraperitoneal Radioimmunotherapy
- PMID: 35798559
- PMCID: PMC9841246
- DOI: 10.2967/jnumed.121.263349
Estimating the Risk for Secondary Cancer After Targeted α-Therapy with 211At Intraperitoneal Radioimmunotherapy
Abstract
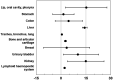
Intraperitoneal 211At-based targeted α-therapy (TAT) may hold great promise as an adjuvant therapy after surgery and chemotherapy in epithelial ovarian cancer to eradicate any remaining undetectable disease. This implies that it will also be delivered to patients possibly already cured by the primary treatment. An estimate of long-term risks is therefore sought to determine whether the treatment is justified. Methods: Baseline data for risk estimates of α-particle irradiation were collected from published studies on excess cancer induction and mortality for subjects exposed to either 224Ra treatments or Thorotrast contrast agent (25% ThO2 colloid, containing 232Th). Organ dosimetry for 224Ra and Thorotrast irradiation were taken from the literature. These organ-specific risks were then applied to our previously reported dosimetry for intraperitoneal 211At-TAT patients. Results: Risk could be estimated for 10 different organ or organ groups. The calculated excess relative risk per gray (ERR/Gy) could be sorted into 2 groups. The lower-ERR/Gy group, ranging up to a value of approximately 5, included trachea, bronchus, and lung, at 0.52 (95% CI, 0.21-0.82); stomach, at 1.4 (95% CI, -5.0-7.9); lymphoid and hematopoietic system, at 2.17 (95% CI, 1.7-2.7); bone and articular cartilage, at 2.6 (95% CI, 2.0-3.3); breast, at 3.45 (95% CI, -10-17); and colon, at 4.5 (95% CI, -3.5-13). The higher-ERR/Gy group, ranging from approximately 10 to 15, included urinary bladder, at 10.1 (95% CI, 1.4-23); liver, at 14.2 (95% CI, 13-16); kidney, at 14.9 (95% CI, 3.9-26); and lip, oral cavity, and pharynx, at 15.20 (95% CI, 2.73-27.63). Applying a typical candidate patient (female, age 65 y) and correcting for the reference population mortality rate, the total estimated excess mortality for an intraperitoneal 211At-monoclonal antibody treatment amounted to 1.13 per 100 treated. More than half this excess originated from urinary bladder and kidney, 0.29 and 0.34, respectively. Depending on various adjustments in calculation and assumptions on competing risks, excess mortality could range from 0.11 to 1.84 per 100 treated. Conclusion: Published epidemiologic data on lifelong detriment after α-particle irradiation and its dosimetry allowed calculations to estimate the risk for secondary cancer after 211At-based intraperitoneal TAT. Measures to reduce dose to the urinary organs may further decrease the estimated relative low risk for secondary cancer from 211At-monoclonal antibody-based intraperitoneal TAT.
Keywords: astatine-211; human; radium-224; secondary cancer; targeted α-therapy; α-particle.
© 2023 by the Society of Nuclear Medicine and Molecular Imaging.
Figures
References
-
- Andersson H, Cederkrantz E, Back T, et al. . Intraperitoneal α-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of 211At-MX35 F(ab′)2—a phase I study. J Nucl Med. 2009;50:1153–1160. - PubMed
-
- Cederkrantz E, Andersson H, Bernhardt P, et al. . Absorbed doses and risk estimates of 211At-MX35 F(ab′)2 in intraperitoneal therapy of ovarian cancer patients. Int J Radiat Oncol Biol Phys. 2015;93:569–576. - PubMed
-
- Becker N, Liebermann D, Wesch H, Van Kaick G. Mortality among Thorotrast-exposed patients and an unexposed comparison group in the German Thorotrast study. Eur J Cancer. 2008;44:1259–1268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical