Deep brain stimulation for Parkinson's disease
- PMID: 35798568
- PMCID: PMC9796446
- DOI: 10.1111/joim.13541
Deep brain stimulation for Parkinson's disease
Abstract
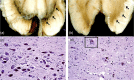
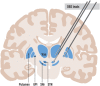
Parkinson's disease (PD) is a progressive neurodegenerative illness with both motor and nonmotor symptoms. Deep brain stimulation (DBS) is an established safe neurosurgical symptomatic therapy for eligible patients with advanced disease in whom medical treatment fails to provide adequate symptom control and good quality of life, or in whom dopaminergic medications induce severe side effects such as dyskinesias. DBS can be tailored to the patient's symptoms and targeted to various nodes along the basal ganglia-thalamus circuitry, which mediates the various symptoms of the illness; DBS in the thalamus is most efficient for tremors, and DBS in the pallidum most efficient for rigidity and dyskinesias, whereas DBS in the subthalamic nucleus (STN) can treat both tremors, akinesia, rigidity and dyskinesias, and allows for decrease in doses of medications even in patients with advanced stages of the disease, which makes it the preferred target for DBS. However, DBS in the STN assumes that the patient is not too old, with no cognitive decline or relevant depression, and does not exhibit severe and medically resistant axial symptoms such as balance and gait disturbances, and falls. Dysarthria is the most common side effect of DBS, regardless of the brain target. DBS has a long-lasting effect on appendicular symptoms, but with progression of disease, nondopaminergic axial features become less responsive to DBS. DBS for PD is highly specialised; to enable adequate selection and follow-up of patients, DBS requires dedicated multidisciplinary teams of movement disorder neurologists, functional neurosurgeons, specialised DBS nurses and neuropsychologists.
Keywords: Parkinson's disease; deep brain stimulation; globus pallidus; pallidotomy; subthalamic nucleus; thalamotomy.
© 2022 The Authors. Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
Marwan Hariz has received honoraria from Boston Scientific for lecturing at meetings. Patric Blomstedt is a consultant for Medtronic, Abbott and Boston Scientific, and a shareholder in Mithridaticum.
Figures

References
-
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–66. - PubMed
-
- Lindvall O. Clinical translation of stem cell transplantation in Parkinson's disease (review). J Intern Med. 2016;279:30–40. - PubMed
-
- Katz M, Luciano MS, Carlson K, Luo P, Marks WJ Jr, Larson PS, et al. Differential effects of deep brain stimulation target on motor subtypes in Parkinson's disease. Ann Neuro. 2015;77:710–9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

