Sex-specific regulation of inhibition and network activity by local aromatase in the mouse hippocampus
- PMID: 35798748
- PMCID: PMC9262915
- DOI: 10.1038/s41467-022-31635-3
Sex-specific regulation of inhibition and network activity by local aromatase in the mouse hippocampus
Abstract
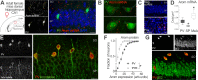
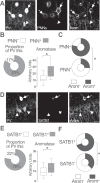
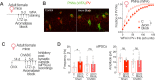
Cognitive function relies on a balanced interplay between excitatory and inhibitory neurons (INs), but the impact of estradiol on IN function is not fully understood. Here, we characterize the regulation of hippocampal INs by aromatase, the enzyme responsible for estradiol synthesis, using a combination of molecular, genetic, functional and behavioral tools. The results show that CA1 parvalbumin-expressing INs (PV-INs) contribute to brain estradiol synthesis. Brain aromatase regulates synaptic inhibition through a mechanism that involves modification of perineuronal nets enwrapping PV-INs. In the female brain, aromatase modulates PV-INs activity, the dynamics of network oscillations and hippocampal-dependent memory. Aromatase regulation of PV-INs and inhibitory synapses is determined by the gonads and independent of sex chromosomes. These results suggest PV-INs are mediators of estrogenic regulation of behaviorally-relevant activity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Garcia-Segura, L. M. Hormones and Brain Plasticity (Oxford University Press, 2009).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

