Impact of walking speed and motion adaptation on optokinetic nystagmus-like head movements in the blowfly Calliphora
- PMID: 35799051
- PMCID: PMC9262929
- DOI: 10.1038/s41598-022-15740-3
Impact of walking speed and motion adaptation on optokinetic nystagmus-like head movements in the blowfly Calliphora
Abstract
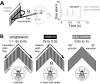
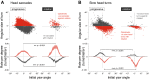
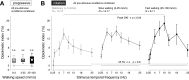
The optokinetic nystagmus is a gaze-stabilizing mechanism reducing motion blur by rapid eye rotations against the direction of visual motion, followed by slower syndirectional eye movements minimizing retinal slip speed. Flies control their gaze through head turns controlled by neck motor neurons receiving input directly, or via descending neurons, from well-characterized directional-selective interneurons sensitive to visual wide-field motion. Locomotion increases the gain and speed sensitivity of these interneurons, while visual motion adaptation in walking animals has the opposite effects. To find out whether flies perform an optokinetic nystagmus, and how it may be affected by locomotion and visual motion adaptation, we recorded head movements of blowflies on a trackball stimulated by progressive and rotational visual motion. Flies flexibly responded to rotational stimuli with optokinetic nystagmus-like head movements, independent of their locomotor state. The temporal frequency tuning of these movements, though matching that of the upstream directional-selective interneurons, was only mildly modulated by walking speed or visual motion adaptation. Our results suggest flies flexibly control their gaze to compensate for rotational wide-field motion by a mechanism similar to an optokinetic nystagmus. Surprisingly, the mechanism is less state-dependent than the response properties of directional-selective interneurons providing input to the neck motor system.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Optokinetic eye movements elicited by radial optic flow in the macaque monkey.J Neurophysiol. 1998 Mar;79(3):1461-80. doi: 10.1152/jn.1998.79.3.1461. J Neurophysiol. 1998. PMID: 9497425
-
Development of optokinetic neuronal responses in the pretectum and horizontal optokinetic nystagmus in unilaterally enucleated rats.Arch Ital Biol. 1989 Oct;127(4):225-42. Arch Ital Biol. 1989. PMID: 2604504
-
Comparison of optomotor and optokinetic reflexes in mice.J Neurophysiol. 2017 Jul 1;118(1):300-316. doi: 10.1152/jn.00055.2017. Epub 2017 Apr 19. J Neurophysiol. 2017. PMID: 28424291 Free PMC article.
-
The relationship between infantile strabismus and latent nystagmus.Eye (Lond). 1996;10 ( Pt 2):274-81. doi: 10.1038/eye.1996.58. Eye (Lond). 1996. PMID: 8776460 Review.
-
The sensing of rotational and translational optic flow by the primate optokinetic system.Rev Oculomot Res. 1993;5:393-403. Rev Oculomot Res. 1993. PMID: 8420560 Review.
Cited by
-
Optokinetic response in D. melanogaster reveals the nature of common repellent odorants.Sci Rep. 2024 Sep 27;14(1):22277. doi: 10.1038/s41598-024-73221-1. Sci Rep. 2024. PMID: 39333197 Free PMC article.
References
-
- Walls GL. The evolutionary history of eye movements. Vis. Res. 1962;2:69–80. doi: 10.1016/0042-6989(62)90064-0. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

