Comparison of cone bioassay estimates at two laboratories with different Anopheles mosquitoes for quality assurance of pyrethroid insecticide-treated nets
- PMID: 35799172
- PMCID: PMC9264565
- DOI: 10.1186/s12936-022-04217-3
Comparison of cone bioassay estimates at two laboratories with different Anopheles mosquitoes for quality assurance of pyrethroid insecticide-treated nets
Abstract
Background: Quality assurance (QA) of insecticide-treated nets (ITNs) delivered to malaria-endemic countries is conducted by measuring physiochemical parameters, but not bioefficacy against malaria mosquitoes. This study explored utility of cone bioassays for pre-delivery QA of pyrethroid ITNs to test the assumption that cone bioassays are consistent across locations, mosquito strains, and laboratories.
Methods: Double-blinded bioassays were conducted on twenty unused pyrethroid ITNs of 4 brands (100 nets, 5 subsamples per net) that had been delivered for mass distribution in Papua New Guinea (PNG) having passed predelivery inspections. Cone bioassays were performed on the same net pieces following World Health Organization (WHO) guidelines at the PNG Institute of Medical Research (PNGIMR) using pyrethroid susceptible Anopheles farauti sensu stricto (s.s.) and at Ifakara Health Institute (IHI), Tanzania using pyrethroid susceptible Anopheles gambiae s.s. Additionally, WHO tunnel tests were conducted at IHI on ITNs that did not meet cone bioefficacy thresholds. Results from IHI and PNGIMR were compared using Spearman's Rank correlation, Bland-Altman (BA) analysis and analysis of agreement. Literature review on the use of cone bioassays for unused pyrethroid ITNs testing was conducted.
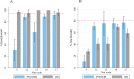
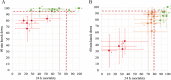
Results: In cone bioassays, 13/20 nets (65%) at IHI and 8/20 (40%) at PNGIMR met WHO bioefficacy criteria. All nets met WHO bioefficacy criteria on combined cone/tunnel tests at IHI. Results from IHI and PNGIMR correlated on 60-min knockdown (KD60) (rs = 0.6,p = 0.002,n = 20) and 24-h mortality (M24) (rs = 0.9,p < 0.0001,n = 20) but BA showed systematic bias between the results. Of the 5 nets with discrepant result between IHI and PNGIMR, three had confidence intervals overlapping the 80% mortality threshold, with averages within 1-3% of the threshold. Including these as a pass, the agreement between the results to predict ITN failure was good with kappa = 0.79 (0.53-1.00) and 90% accuracy.
Conclusions: Based on these study findings, the WHO cone bioassay is a reproducible bioassay for ITNs with > 80% M24, and for all ITNs provided inherent stochastic variation and systematic bias are accounted for. The literature review confirms that WHO cone bioassay bioefficacy criteria have been previously achieved by all pyrethroid ITNs (unwashed), without the need for additional tunnel tests. The 80% M24 threshold remains the most reliable indicator of pyrethroid ITN quality using pyrethroid susceptible mosquitoes. In the absence of alternative tests, cone bioassays could be used as part of pre-delivery QA.
Keywords: Anopheles; Bioassay; Bioefficacy; Cone bioassay; ITN; Insecticide treated nets; LLIN; Long lasting insecticidal nets; Malaria; Mosquito; Pyrethroid; Quality assurance; Tunnel test.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests. SJM, EM, JM, KM, OGO evaluate vector control products including ITNs for a number of manufacturers including the manufacturers of several ITNs reported in this article. No manufacturers of ITNs had any input into the article.
Figures




Similar articles
-
Comparing the new Ifakara Ambient Chamber Test with WHO cone and tunnel tests for bioefficacy and non-inferiority testing of insecticide-treated nets.Malar J. 2019 Apr 30;18(1):153. doi: 10.1186/s12936-019-2741-y. Malar J. 2019. PMID: 31039788 Free PMC article.
-
WHO cone bioassay boards with or without holes: relevance for bioassay outcomes in long-lasting insecticidal net studies.Malar J. 2022 Dec 19;21(1):389. doi: 10.1186/s12936-022-04412-2. Malar J. 2022. PMID: 36536444 Free PMC article.
-
Influence of testing modality on bioefficacy for the evaluation of Interceptor® G2 mosquito nets to combat malaria mosquitoes in Tanzania.Parasit Vectors. 2022 Apr 11;15(1):124. doi: 10.1186/s13071-022-05207-9. Parasit Vectors. 2022. PMID: 35410250 Free PMC article.
-
Indoor residual spraying for preventing malaria in communities using insecticide-treated nets.Cochrane Database Syst Rev. 2019 May 23;5(5):CD012688. doi: 10.1002/14651858.CD012688.pub2. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2022 Jan 17;1:CD012688. doi: 10.1002/14651858.CD012688.pub3. PMID: 31120132 Free PMC article. Updated.
-
From the factory to the field: considerations of product characteristics for insecticide-treated net (ITN) bioefficacy testing.Malar J. 2021 Sep 6;20(1):363. doi: 10.1186/s12936-021-03897-7. Malar J. 2021. PMID: 34488778 Free PMC article. Review.
Cited by
-
A bioassay method validation framework for laboratory and semi-field tests used to evaluate vector control tools.Malar J. 2023 Sep 28;22(1):289. doi: 10.1186/s12936-023-04717-w. Malar J. 2023. PMID: 37770855 Free PMC article.
-
Coating formulation change leads to inferior performance of long-lasting insecticidal nets in Papua New Guinea.Malar J. 2022 Nov 24;21(1):349. doi: 10.1186/s12936-022-04392-3. Malar J. 2022. PMID: 36424604 Free PMC article.
-
Time of exposure and assessment influence the mortality induced by insecticides against metabolic resistant mosquitoes.Parasit Vectors. 2024 Mar 2;17(1):103. doi: 10.1186/s13071-024-06190-z. Parasit Vectors. 2024. PMID: 38431631 Free PMC article.
-
Temperature, mosquito feeding status and mosquito density influence the measured bio-efficacy of insecticide-treated nets in cone assays.Parasit Vectors. 2024 Mar 28;17(1):159. doi: 10.1186/s13071-024-06210-y. Parasit Vectors. 2024. PMID: 38549097 Free PMC article.
-
Complete series method (CSM): a convenient method to reduce daily heterogeneity when evaluating the regeneration time (RT) of insecticide-treated nets (ITNs).Parasit Vectors. 2024 May 22;17(1):235. doi: 10.1186/s13071-024-06323-4. Parasit Vectors. 2024. PMID: 38778423 Free PMC article.
References
-
- WHO . Guidelines for malaria. Geneva: World Health Organization; 2021.
-
- WHO . Global report on insecticide resistance in malaria vectors. Geneva: World Health Organization; 2018.
-
- Kleinschmidt I, Bradley J, Knox TB, Mnzava AP, Kafy HT, Mbogo C, et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: a WHO-coordinated, prospective, international, observational cohort study. Lancet Infect Dis. 2018;18:640–649. doi: 10.1016/S1473-3099(18)30172-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

