A booster dose of Delta × Omicron hybrid mRNA vaccine produced broadly neutralizing antibody against Omicron and other SARS-CoV-2 variants
- PMID: 35799178
- PMCID: PMC9261010
- DOI: 10.1186/s12929-022-00830-1
A booster dose of Delta × Omicron hybrid mRNA vaccine produced broadly neutralizing antibody against Omicron and other SARS-CoV-2 variants
Abstract
Background: With the continuous emergence of new SARS-CoV-2 variants that feature increased transmission and immune escape, there is an urgent demand for a better vaccine design that will provide broader neutralizing efficacy.
Methods: We report an mRNA-based vaccine using an engineered "hybrid" receptor binding domain (RBD) that contains all 16 point-mutations shown in the currently prevailing Omicron and Delta variants.
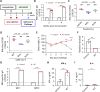
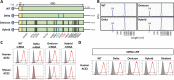
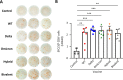
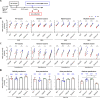
Results: A booster dose of hybrid vaccine in mice previously immunized with wild-type RBD vaccine induced high titers of broadly neutralizing antibodies against all tested SARS-CoV-2 variants of concern (VOCs). In naïve mice, hybrid vaccine generated strong Omicron-specific neutralizing antibodies as well as low but significant titers against other VOCs. Hybrid vaccine also elicited CD8+/IFN-γ+ T cell responses against a conserved T cell epitope present in wild type and all VOCs.
Conclusions: These results demonstrate that inclusion of different antigenic mutations from various SARS-CoV-2 variants is a feasible approach to develop cross-protective vaccines.
Keywords: Booster dose; COVID-19; Cross-protectivity; Hybrid vaccine; Next generation vaccine; Omicron vaccine; SARS-CoV-2; Variants of concern; mRNA vaccine.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, Peacock SJ, COVID-19 Genomics UK (COG-UK) Consortium. Robertson DL. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. - DOI - PMC - PubMed
-
- Planas D, Bruel T, Grzelak L, Guivel-Benhassine F, Staropoli I, Porrot F, Planchais C, Buchrieser J, Rajah MM, Bishop E, Albert M, Donati F, Prot M, Behillil S, Enouf V, Maquart M, Smati-Lafarge M, Varon E, Schortgen F, Yahyaoui L, Gonzalez M, De Seze J, Pere H, Veyer D, Seve A, Simon-Loriere E, Fafi-Kremer S, Stefic K, Mouquet H, Hocqueloux L, van der Werf S, Prazuck T, Schwartz O. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27(5):917–924. doi: 10.1038/s41591-021-01318-5. - DOI - PubMed
-
- Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira I, Datir R, Collier DA, Albecka A, Singh S, Pandey R, Brown J, Zhou J, Goonawardane N, Mishra S, Whittaker C, Mellan T, Marwal R, Datta M, Sengupta S, Ponnusamy K, Radhakrishnan VS, Abdullahi A, Charles O, Chattopadhyay P, Devi P, Caputo D, Peacock T, Wattal C, Goel N, Satwik A, Vaishya R, Agarwal M, The Genotype to Phenotype Japan (G2P-Japan) Consortium. The CITIID-NIHR BioResource COVID-19 Collaboration. Mavousian A, Lee JH, Bassi J, Silacci-Fegni C, Saliba C, Pinto D, Irie T, Yoshida I, Hamilton WL, Sato K, Bhatt S, Flaxman S, James LC, Corti D, Piccoli L, Barclay WS, Rakshit P, Agrawal A, Gupta RK. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599(7883):114–119. doi: 10.1038/s41586-021-03944-y. - DOI - PMC - PubMed
-
- Twohig KA, Nyberg T, Zaidi A, Thelwall S, Sinnathamby MA, Aliabadi S, Seaman SR, Harris RJ, Hope R, Lopez-Bernal J, Gallagher E, Charlett A, De Angelis D, Presanis AM, Dabrera G, COVID-19 Genomics UK (COG-UK) consortium Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22(1):35–42. doi: 10.1016/S1473-3099(21)00475-8. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

