Recent progress on vascular endothelial growth factor receptor inhibitors with dual targeting capabilities for tumor therapy
- PMID: 35799213
- PMCID: PMC9263050
- DOI: 10.1186/s13045-022-01310-7
Recent progress on vascular endothelial growth factor receptor inhibitors with dual targeting capabilities for tumor therapy
Abstract
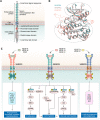
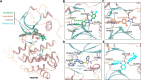
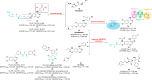
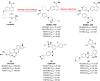
Vascular endothelial growth factor receptors (VEGFRs) are a family of receptor protein tyrosine kinases that play an important role in the regulation of tumor-induced angiogenesis. Currently, VEGFR inhibitors have been widely used in the treatment of various tumors. However, current VEGFR inhibitors are limited to a certain extent due to limited clinical efficacy and potential toxicity, which hinder their clinical application. Thus, the development of new strategies to improve the clinical outcomes and minimize the toxic effects of VEGFR inhibitors is required. Given the synergistic effect of VEGFR and other therapies in tumor development and progression, VEGFR dual-target inhibitors are becoming an attractive approach due to their favorable pharmacodynamics, low toxicity, and anti-resistant effects. This perspective provides an overview of the development of VEGFR dual-target inhibitors from multiple aspects, including rational target combinations, drug discovery strategies, structure-activity relationships and future directions.
Keywords: Antiangiogenesis treatment; Antitumor drugs; Dual inhibitor; VEGFR kinase.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3.Clin Cancer Res. 2008 Nov 15;14(22):7272-83. doi: 10.1158/1078-0432.CCR-08-0652. Clin Cancer Res. 2008. PMID: 19010843
-
KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties.Cancer Res. 2006 Sep 15;66(18):9134-42. doi: 10.1158/0008-5472.CAN-05-4290. Cancer Res. 2006. PMID: 16982756
-
Selective VEGFR inhibitors for anticancer therapeutics in clinical use and clinical trials.Curr Pharm Des. 2012;18(20):2921-35. doi: 10.2174/138161212800672732. Curr Pharm Des. 2012. PMID: 22571661 Review.
-
Anti-angiogenic Agents: A Review on Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2) Inhibitors.Curr Med Chem. 2021;28(13):2540-2564. doi: 10.2174/0929867327666200514082425. Curr Med Chem. 2021. PMID: 32407259 Review.
-
Recent development of multi-target VEGFR-2 inhibitors for the cancer therapy.Bioorg Chem. 2023 Apr;133:106425. doi: 10.1016/j.bioorg.2023.106425. Epub 2023 Feb 15. Bioorg Chem. 2023. PMID: 36801788 Review.
Cited by
-
An Overview of Renal Cell Carcinoma Hallmarks, Drug Resistance, and Adjuvant Therapies.Cancer Diagn Progn. 2023 Nov 3;3(6):616-634. doi: 10.21873/cdp.10264. eCollection 2023 Nov-Dec. Cancer Diagn Progn. 2023. PMID: 37927802 Free PMC article. Review.
-
Identification of VEGFs-related gene signature for predicting microangiogenesis and hepatocellular carcinoma prognosis.Aging (Albany NY). 2024 Jun 13;16(12):10321-10347. doi: 10.18632/aging.205931. Epub 2024 Jun 13. Aging (Albany NY). 2024. PMID: 38874512 Free PMC article.
-
CYP1B1 promotes angiogenesis and sunitinib resistance in clear cell renal cell carcinoma via USP5-mediated HIF2α deubiquitination.Neoplasia. 2025 Aug;66:101186. doi: 10.1016/j.neo.2025.101186. Epub 2025 May 27. Neoplasia. 2025. PMID: 40435846 Free PMC article. Review.
-
Targeting hyperactive platelet-derived growth factor receptor-β signaling in T-cell acute lymphoblastic leukemia and lymphoma.Haematologica. 2024 May 1;109(5):1373-1384. doi: 10.3324/haematol.2023.283981. Haematologica. 2024. PMID: 37941480 Free PMC article.
-
Targeting the tumor stroma for cancer therapy.Mol Cancer. 2022 Nov 2;21(1):208. doi: 10.1186/s12943-022-01670-1. Mol Cancer. 2022. PMID: 36324128 Free PMC article. Review.
References
-
- Li Y, Yang G, Zhang J, Tang P, Yang C, Wang G, Chen J, Liu J, Zhang L, Ouyang L. Discovery, synthesis, and evaluation of highly selective vascular endothelial growth factor receptor 3 (VEGFR3) inhibitor for the potential treatment of metastatic triple-negative breast cancer. J Med Chem. 2021;64:12022–12048. doi: 10.1021/acs.jmedchem.1c00678. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2019YFS0003/Sichuan Science and Technology Program
- 2018ZX09201018-021/National Major Scientific and Technological Special Project for Significant New Drugs Development
- 81903502/National Natural Science Foundation of China
- 81922064/National Natural Science Foundation of China
- 81874290/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

