Transplantation of bacteriophages from ulcerative colitis patients shifts the gut bacteriome and exacerbates the severity of DSS colitis
- PMID: 35799219
- PMCID: PMC9264660
- DOI: 10.1186/s40168-022-01275-2
Transplantation of bacteriophages from ulcerative colitis patients shifts the gut bacteriome and exacerbates the severity of DSS colitis
Abstract
Background: Inflammatory bowel diseases (IBDs) including Crohn's disease (CD) and ulcerative colitis (UC) are characterized by chronic and debilitating gut inflammation. Altered bacterial communities of the intestine are strongly associated with IBD initiation and progression. The gut virome, which is primarily composed of bacterial viruses (bacteriophages, phages), is thought to be an important factor regulating and shaping microbial communities in the gut. While alterations in the gut virome have been observed in IBD patients, the contribution of these viruses to alterations in the bacterial community and heightened inflammatory responses associated with IBD patients remains largely unknown.
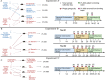
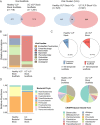
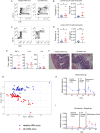
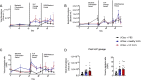
Results: Here, we performed in vivo microbial cross-infection experiments to follow the effects of fecal virus-like particles (VLPs) isolated from UC patients and healthy controls on bacterial diversity and severity of experimental colitis in human microbiota-associated (HMA) mice. Shotgun metagenomics confirmed that several phages were transferred to HMA mice, resulting in treatment-specific alterations in the gut virome. VLPs from healthy and UC patients also shifted gut bacterial diversity of these mice, an effect that was amplified during experimental colitis. VLPs isolated from UC patients specifically altered the relative abundance of several bacterial taxa previously implicated in IBD progression. Additionally, UC VLP administration heightened colitis severity in HMA mice, as indicated by shortened colon length and increased pro-inflammatory cytokine production. Importantly, this effect was dependent on intact VLPs.
Conclusions: Our findings build on recent literature indicating that phages are dynamic regulators of bacterial communities in the gut and implicate the intestinal virome in modulating intestinal inflammation and disease. Video Abstract.
Keywords: Bacteriophages; DSS colitis; Inflammatory bowel disease; Intestine; Microbiota; Ulcerative colitis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Gut virome-wide association analysis identifies cross-population viral signatures for inflammatory bowel disease.Microbiome. 2024 Jul 18;12(1):130. doi: 10.1186/s40168-024-01832-x. Microbiome. 2024. PMID: 39026313 Free PMC article.
-
Whole-Virome Analysis Sheds Light on Viral Dark Matter in Inflammatory Bowel Disease.Cell Host Microbe. 2019 Dec 11;26(6):764-778.e5. doi: 10.1016/j.chom.2019.10.009. Epub 2019 Nov 19. Cell Host Microbe. 2019. PMID: 31757768
-
2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-glucoside, a major bioactive component from Polygoni multiflori Radix (Heshouwu) suppresses DSS induced acute colitis in BALb/c mice by modulating gut microbiota.Biomed Pharmacother. 2021 May;137:111420. doi: 10.1016/j.biopha.2021.111420. Epub 2021 Feb 23. Biomed Pharmacother. 2021. PMID: 33761623
-
Gut virome and its implications in the pathogenesis and therapeutics of inflammatory bowel disease.BMC Med. 2025 Mar 26;23(1):183. doi: 10.1186/s12916-025-04016-y. BMC Med. 2025. PMID: 40140901 Free PMC article. Review.
-
Microbiome-phage interactions in inflammatory bowel disease.Clin Microbiol Infect. 2023 Jun;29(6):682-688. doi: 10.1016/j.cmi.2022.08.027. Epub 2022 Oct 1. Clin Microbiol Infect. 2023. PMID: 36191844 Review.
Cited by
-
The role of complex interactions between the intestinal flora and host in regulating intestinal homeostasis and inflammatory bowel disease.Front Microbiol. 2023 Jun 14;14:1188455. doi: 10.3389/fmicb.2023.1188455. eCollection 2023. Front Microbiol. 2023. PMID: 37389342 Free PMC article. Review.
-
Intratumoural microbiota: a new frontier in cancer development and therapy.Signal Transduct Target Ther. 2024 Jan 10;9(1):15. doi: 10.1038/s41392-023-01693-0. Signal Transduct Target Ther. 2024. PMID: 38195689 Free PMC article. Review.
-
Revived ancient viruses from deep-sea ecosystems are biothreats by triggering gut dysbiosis.mBio. 2025 Aug 13;16(8):e0121725. doi: 10.1128/mbio.01217-25. Epub 2025 Jul 11. mBio. 2025. PMID: 40643250 Free PMC article.
-
Convergence of gut phage communities but not bacterial communities following wild mouse bacteriophage transplantation into captive house mice.ISME J. 2024 Jan 8;18(1):wrae178. doi: 10.1093/ismejo/wrae178. ISME J. 2024. PMID: 39276368 Free PMC article.
-
Benchmarking of a time-saving and scalable protocol for the extraction of DNA from diverse viromes.PeerJ. 2025 Jan 14;13:e18785. doi: 10.7717/peerj.18785. eCollection 2025. PeerJ. 2025. PMID: 39830964 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

