Interleukin-1: an important target for perinatal neuroprotection?
- PMID: 35799507
- PMCID: PMC9241389
- DOI: 10.4103/1673-5374.341044
Interleukin-1: an important target for perinatal neuroprotection?
Abstract
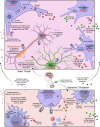
Perinatal inflammation is a significant risk factor for lifelong neurodevelopmental impairments such as cerebral palsy. Extensive clinical and preclinical evidence links the severity and pattern of perinatal inflammation to impaired maturation of white and grey matters and reduced brain growth. Multiple pathways are involved in the pathogenesis of perinatal inflammation. However, studies of human and experimental perinatal encephalopathy have demonstrated a strong causative link between perinatal encephalopathy and excessive production of the pro-inflammatory effector cytokine interleukin-1. In this review, we summarize clinical and preclinical evidence that underpins interleukin-1 as a critical factor in initiating and perpatuating systemic and central nervous system inflammation and subsequent perinatal brain injury. We also highlight the important role of endogenous interleukin-1 receptor antagonist in mitigating interleukin-1-driven neuroinflammation and tissue damage, and summarize outcomes from clinical and mechanistic animal studies that establish the commercially available interleukin-1 receptor antagonist, anakinra, as a safe and effective therapeutic intervention. We reflect on the evidence supporting clinical translation of interleukin-1 receptor antagonist for infants at the greatest risk of perinatal inflammation and impaired neurodevelopment, and suggest a path to advance interleukin-1 receptor antagonist along the translational path for perinatal neuroprotection.
Keywords: brain; inflammation; interleukin-1; interleukin-1 receptor antagonist; interleukin-1β; neonatal encephalopathy; neuroprotection; preterm brain injury.
Conflict of interest statement
Figures
Similar articles
-
Microglia-Mediated Neurodegeneration in Perinatal Brain Injuries.Biomolecules. 2021 Jan 13;11(1):99. doi: 10.3390/biom11010099. Biomolecules. 2021. PMID: 33451166 Free PMC article. Review.
-
Neuronal self-injury mediated by IL-1β and MMP-9 in a cerebral palsy model of severe neonatal encephalopathy induced by immune activation plus hypoxia-ischemia.J Neuroinflammation. 2015 May 30;12:111. doi: 10.1186/s12974-015-0330-8. J Neuroinflammation. 2015. PMID: 26025257 Free PMC article.
-
A Systematic Review of Magnesium Sulfate for Perinatal Neuroprotection: What Have We Learnt From the Past Decade?Front Neurol. 2020 May 27;11:449. doi: 10.3389/fneur.2020.00449. eCollection 2020. Front Neurol. 2020. PMID: 32536903 Free PMC article.
-
[Oxytocin: a new target for neuroprotection?].Biol Aujourdhui. 2022;216(3-4):145-153. doi: 10.1051/jbio/2022012. Epub 2023 Feb 6. Biol Aujourdhui. 2022. PMID: 36744980 French.
-
Involvement of neuronal IL-1β in acquired brain lesions in a rat model of neonatal encephalopathy.J Neuroinflammation. 2013 Sep 5;10:110. doi: 10.1186/1742-2094-10-110. J Neuroinflammation. 2013. PMID: 24007297 Free PMC article.
Cited by
-
Progressive inflammation reduces high-frequency EEG activity and cortical dendritic arborisation in late gestation fetal sheep.J Neuroinflammation. 2023 May 24;20(1):124. doi: 10.1186/s12974-023-02805-x. J Neuroinflammation. 2023. PMID: 37226206 Free PMC article.
-
Intra-amniotic infection with Ureaplasma parvum causes serovar-dependent white matter damage in preterm fetal sheep.Brain Commun. 2025 May 24;7(3):fcaf182. doi: 10.1093/braincomms/fcaf182. eCollection 2025. Brain Commun. 2025. PMID: 40462859 Free PMC article.
-
The role of maternal infections in neurodevelopmental psychiatric disorders: focus on the P2X7/NLRP3/IL-1β signalling pathway.J Neuroinflammation. 2025 Jul 26;22(1):192. doi: 10.1186/s12974-025-03509-0. J Neuroinflammation. 2025. PMID: 40713568 Free PMC article. Review.
-
What is the pathophysiology of inflammation-induced cortical injury in the perinatal brain?Neural Regen Res. 2026 Feb 1;21(2):502-505. doi: 10.4103/NRR.NRR-D-24-01091. Epub 2025 Jan 29. Neural Regen Res. 2026. PMID: 39885678 Free PMC article.
-
Anakinra Pilot - a clinical trial to demonstrate safety, feasibility and pharmacokinetics of interleukin 1 receptor antagonist in preterm infants.Front Immunol. 2022 Oct 27;13:1022104. doi: 10.3389/fimmu.2022.1022104. eCollection 2022. Front Immunol. 2022. PMID: 36389766 Free PMC article. Clinical Trial.
References
-
- Baier RJ. Genetics of perinatal brain injury in the preterm infant. Front Biosci. 2006;11:1371–1387. - PubMed
-
- Barlow RM. The foetal sheep:morphogenesis of the nervous system and histochemical aspects of myelination. J Comp Neurol. 1969;135:249–262. - PubMed
-
- Bartha AI, Foster-Barber A, Miller SP, Vigneron DB, Glidden DV, Barkovich AJ, Ferriero DM. Neonatal encephalopathy:association of cytokines with MR spectroscopy and outcome. Pediatr Res. 2004;56:960–966. - PubMed
-
- Beaudry-Richard A, Nadeau-Vallée M, Prairie É, Maurice N, Heckel É, Nezhady M, Pundir S, Madaan A, Boudreault A, Hou X, Quiniou C, Sierra EM, Beaulac A, Lodygensky G, Robertson SA, Keelan J, Adams Waldorf KM, Olson DM, Rivera JC, et al. Antenatal IL-1-dependent inflammation persists postnatally and causes retinal and sub-retinal vasculopathy in progeny. Sci Rep. 2018;8:11875. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

