The regulatory role of Pin1 in neuronal death
- PMID: 35799512
- PMCID: PMC9241412
- DOI: 10.4103/1673-5374.341043
The regulatory role of Pin1 in neuronal death
Abstract
Regulated cell death predominantly involves apoptosis, autophagy, and regulated necrosis. It is vital that we understand how key regulatory signals can control the process of cell death. Pin1 is a cis-trans isomerase that catalyzes the isomerization of phosphorylated serine or threonine-proline motifs of a protein, thereby acting as a crucial molecular switch and regulating the protein functionality and the signaling pathways involved. However, we know very little about how Pin1-associated pathways might play a role in regulated cell death. In this paper, we review the role of Pin1 in regulated cell death and related research progress and summarize Pin1-related pathways in regulated cell death. Aside from the involvement of Pin1 in the apoptosis that accompanies neurodegenerative diseases, accumulating evidence suggests that Pin1 also plays a role in regulated necrosis and autophagy, thereby exhibiting distinct effects, including both neurotoxic and neuroprotective effects. Gaining an enhanced understanding of Pin1 in neuronal death may provide us with new options for the development of therapeutic target for neurodegenerative disorders.
Keywords: Pin1; apoptosis; autophagy; calpain; central nervous system; necroptosis; necrosis; neurodegenerative diseases; neuron; regulated neuronal death.
Conflict of interest statement
Figures
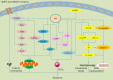
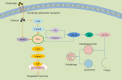
Similar articles
-
The Peptidyl-prolyl Isomerase Pin1 in Neuronal Signaling: from Neurodevelopment to Neurodegeneration.Mol Neurobiol. 2021 Mar;58(3):1062-1073. doi: 10.1007/s12035-020-02179-8. Epub 2020 Oct 21. Mol Neurobiol. 2021. PMID: 33083964 Free PMC article. Review.
-
Role of phosphorylation in determining the backbone dynamics of the serine/threonine-proline motif and Pin1 substrate recognition.Biochemistry. 1998 Apr 21;37(16):5566-75. doi: 10.1021/bi973060z. Biochemistry. 1998. PMID: 9548941
-
Gears-In-Motion: The Interplay of WW and PPIase Domains in Pin1.Front Oncol. 2018 Oct 25;8:469. doi: 10.3389/fonc.2018.00469. eCollection 2018. Front Oncol. 2018. PMID: 30460195 Free PMC article. Review.
-
Phosphorylation-Dependent Pin1 Isomerization of ATR: Its Role in Regulating ATR's Anti-apoptotic Function at Mitochondria, and the Implications in Cancer.Front Cell Dev Biol. 2020 Apr 30;8:281. doi: 10.3389/fcell.2020.00281. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32426354 Free PMC article. Review.
-
PIN1 in Cell Cycle Control and Cancer.Front Pharmacol. 2018 Nov 26;9:1367. doi: 10.3389/fphar.2018.01367. eCollection 2018. Front Pharmacol. 2018. PMID: 30534074 Free PMC article. Review.
Cited by
-
Pin1 and Alzheimer's disease.Transl Res. 2023 Apr;254:24-33. doi: 10.1016/j.trsl.2022.09.003. Epub 2022 Sep 23. Transl Res. 2023. PMID: 36162703 Free PMC article. Review.
-
A bibliometric analysis of apoptosis in glaucoma.Front Neurosci. 2023 Feb 6;17:1105158. doi: 10.3389/fnins.2023.1105158. eCollection 2023. Front Neurosci. 2023. PMID: 36814788 Free PMC article.
-
Advances in the Research of Mesenchymal Stromal Cells in the Treatment of Maxillofacial Neurological Disorders and the Promotion of Facial Nerve Regeneration.Mol Neurobiol. 2025 Apr 28. doi: 10.1007/s12035-025-04981-8. Online ahead of print. Mol Neurobiol. 2025. PMID: 40295362 Review.
-
Identification of Key Genes and Immune Characteristics of SASP in Acute Ischemic Stroke.J Mol Neurosci. 2025 Feb 17;75(1):22. doi: 10.1007/s12031-025-02312-z. J Mol Neurosci. 2025. PMID: 39960563
-
PEP-1-PIN1 Promotes Hippocampal Neuronal Cell Survival by Inhibiting Cellular ROS and MAPK Phosphorylation.Biomedicines. 2024 Oct 15;12(10):2352. doi: 10.3390/biomedicines12102352. Biomedicines. 2024. PMID: 39457664 Free PMC article.
References
-
- Albayram O, Kondo A, Mannix R, Smith C, Tsai CY, Li C, Herbert MK, Qiu J, Monuteaux M, Driver J, Yan S, Gormley W, Puccio AM, Okonkwo DO, Lucke-Wold B, Bailes J, Meehan W, Zeidel M, Lu KP, Zhou XZ. Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae. Nat Commun. 2017;8:1000. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

