D-galactose Intake Alleviates Atopic Dermatitis in Mice by Modulating Intestinal Microbiota
- PMID: 35799581
- PMCID: PMC9254681
- DOI: 10.3389/fnut.2022.895837
D-galactose Intake Alleviates Atopic Dermatitis in Mice by Modulating Intestinal Microbiota
Abstract
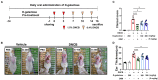
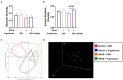
Atopic dermatitis (AD) is one of the most prevalent, chronic and persistent inflammatory skin diseases closely associated with intestinal microbiota. To evaluate the effect of D-galactose intake on AD, we orally administered D-galactose to BALB/c mice whose ears and skin were treated with 2,4-dinitrochlorobenzene (DNCB). D-galactose alleviated DNCB-induced AD-like phenotypes such as redness, scaling/dryness and excoriation. Ear thickness was also decreased by D-galactose administration. Histopathological analysis revealed decreased epidermal thickening, infiltration of immune cells, especially mast cells, in the dermis. Total levels of serum IgE representing the immunological response of AD were decreased by D-galactose administration. Microbiota analysis showed that D-galactose administration restored gut microbiota profiles, which were altered in AD mice, characterized by increased abundance of Bacteroidetes and decreased abundance of Firmicutes. The increased abundance of Bacteroides and the decreased abundance of Prevotella and Ruminococcus were reversed by D-galactose treatment, following improvement of AD. Our results suggest the possible use of D-galactose as a prebiotic to alleviate AD by altering gut microbiota.
Keywords: D-galactose; atopic dermatitis; inflammation; microbiota; nutrition.
Copyright © 2022 Kim, Jung, Song, Jang, Park, Ahn, Lee, Kim, Lee, Seo, Kim, Ryu, Sim and Park.
Conflict of interest statement
H-EK, E-JR, and JS were employed by Quorum Bio Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Extracellular vesicles of Limosilactobacillus fermentum SLAM216 ameliorate skin symptoms of atopic dermatitis by regulating gut microbiome on serotonin metabolism.Gut Microbes. 2025 Dec;17(1):2474256. doi: 10.1080/19490976.2025.2474256. Epub 2025 Mar 3. Gut Microbes. 2025. PMID: 40028723 Free PMC article.
-
Baicalin ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice through modulating skin barrier function, gut microbiota and JAK/STAT pathway.Bioorg Chem. 2022 Feb;119:105538. doi: 10.1016/j.bioorg.2021.105538. Epub 2021 Dec 6. Bioorg Chem. 2022. PMID: 34929516
-
Pediococcus acidilactici intake decreases the clinical severity of atopic dermatitis along with increasing mucin production and improving the gut microbiome in Nc/Nga mice.Biomed Pharmacother. 2020 Sep;129:110488. doi: 10.1016/j.biopha.2020.110488. Epub 2020 Jul 6. Biomed Pharmacother. 2020. PMID: 32768968
-
Phloretin alleviates dinitrochlorobenzene-induced dermatitis in BALB/c mice.Int J Immunopathol Pharmacol. 2020 Jan-Dec;34:2058738420929442. doi: 10.1177/2058738420929442. Int J Immunopathol Pharmacol. 2020. PMID: 32571120 Free PMC article.
-
Topical application of Taglisodog-eum inhibits the development of experimental atopic dermatitis.J Ethnopharmacol. 2013 Jan 30;145(2):536-46. doi: 10.1016/j.jep.2012.11.026. Epub 2012 Dec 2. J Ethnopharmacol. 2013. PMID: 23211659
Cited by
-
The Effects of Saposhnikovia divaricata Aqueous Extracts on the Inflammation and Intestinal Microflora in Allergic Rhinitis Mice.Evid Based Complement Alternat Med. 2022 Oct 14;2022:1052359. doi: 10.1155/2022/1052359. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36276863 Free PMC article.
-
Exploratory Study of Gastrointestinal Redox Biomarkers in the Presymptomatic and Symptomatic Tg2576 Mouse Model of Familial Alzheimer's Disease: Phenotypic Correlates and Effects of Chronic Oral d-Galactose.ACS Chem Neurosci. 2023 Nov 15;14(22):4013-4025. doi: 10.1021/acschemneuro.3c00495. Epub 2023 Nov 6. ACS Chem Neurosci. 2023. PMID: 37932005 Free PMC article.
-
Exposure to chlorinated drinking water alters the murine fecal microbiota.Sci Total Environ. 2024 Mar 1;914:169933. doi: 10.1016/j.scitotenv.2024.169933. Epub 2024 Jan 8. Sci Total Environ. 2024. PMID: 38199366 Free PMC article.
-
The new era of immune skin diseases: Exploring advances in basic research and clinical translations.J Transl Autoimmun. 2024 Jan 12;8:100232. doi: 10.1016/j.jtauto.2024.100232. eCollection 2024 Jun. J Transl Autoimmun. 2024. PMID: 39022635 Free PMC article. No abstract available.
-
Sweet regulation - The emerging immunoregulatory roles of hexoses.J Adv Res. 2025 Mar;69:361-379. doi: 10.1016/j.jare.2024.04.014. Epub 2024 Apr 15. J Adv Res. 2025. PMID: 38631430 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

