miR-29c-5p knockdown reduces inflammation and blood-brain barrier disruption by upregulating LRP6
- PMID: 35799601
- PMCID: PMC8864056
- DOI: 10.1515/med-2022-0438
miR-29c-5p knockdown reduces inflammation and blood-brain barrier disruption by upregulating LRP6
Abstract
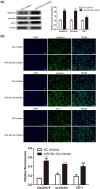
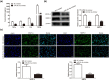
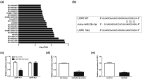
Blood-brain barrier participates in the pathological process of ischemic stroke. MicroRNA-29c-5p was highly expressed in clinical samples from patients with ischemic stroke. In this study, oxygen-glucose deprivation (OGD) treatment of astrocytes enhanced the permeability of brain microvascular endothelial cells (BMECs), and the miR-29c-5p expression was elevated in clinical samples from patients with ischemic stroke. For the function of miR-29c-5p in ischemic stroke, the miR-29c-5p knockdown decreased the permeability and the tight junction protein (TJP) destruction of BMECs and ameliorated the inflammation induced by OGD-treated astrocytes. Mechanistically, miR-29c-5p interacted with lipoprotein receptor-related protein 6 (LRP6) and negatively regulated the LRP6 expression in astrocytes. Moreover, the rescue assays indicated that the interference with miR-29c-5p ameliorated the TJP destruction of BMECs and inflammation caused by OGD-treated astrocytes by increasing the LRP6 expression. Together, miR-29c-5p knockdown decreased the high permeability and the TJP destruction of BMECs and ameliorated the inflammation induced by OGD-treated astrocytes by elevating LRP6 expression.
Keywords: astrocytes; blood–brain barrier; brain microvascular endothelial cells; ischemic stroke; miRNA.
© 2022 Qijun Dai et al., published by De Gruyter.
Conflict of interest statement
Competing interests: The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
LncRNA DANCR attenuates brain microvascular endothelial cell damage induced by oxygen-glucose deprivation through regulating of miR-33a-5p/XBP1s.Aging (Albany NY). 2020 Jan 26;12(2):1778-1791. doi: 10.18632/aging.102712. Epub 2020 Jan 26. Aging (Albany NY). 2020. PMID: 31986122 Free PMC article.
-
Circular RNA circPHC3 Promotes Cell Death and Apoptosis in Human BMECs After Oxygen Glucose Deprivation via miR-455-5p/TRAF3 Axis in vitro.Neuropsychiatr Dis Treat. 2021 Jan 22;17:147-156. doi: 10.2147/NDT.S288669. eCollection 2021. Neuropsychiatr Dis Treat. 2021. PMID: 33519202 Free PMC article.
-
Long Non-coding RNA H19 Deteriorates Hypoxic-Ischemic Brain Damage by Interacting with MicroRNA-140-5p and STAT3.Nanoscale Res Lett. 2022 Apr 5;17(1):43. doi: 10.1186/s11671-022-03666-8. Nanoscale Res Lett. 2022. Retraction in: Discov Nano. 2023 Mar 20;18(1):53. doi: 10.1186/s11671-023-03830-8. PMID: 35380290 Free PMC article. Retracted.
-
Long noncoding RNA MEG3 contributes to dysfunction of brain microvascular endothelial cells after intracerebral hemorrhage by regulating the miR-1930-5p/Mllt1 axis.Brain Res Bull. 2021 Jan;166:1-11. doi: 10.1016/j.brainresbull.2020.10.002. Epub 2020 Oct 27. Brain Res Bull. 2021. Retraction in: Brain Res Bull. 2021 Jun;171:221. doi: 10.1016/j.brainresbull.2021.03.017. PMID: 33127454 Retracted.
-
Serum Exosomal mir-340-5p Promotes Angiogenesis in Brain Microvascular Endothelial Cells During Oxygen-Glucose Deprivation.Neurochem Res. 2022 Apr;47(4):907-920. doi: 10.1007/s11064-021-03492-x. Epub 2022 Jan 7. Neurochem Res. 2022. PMID: 34993704
References
-
- Abdullahi W, Tripathi D, Ronaldson PT. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. 2018;315(3):C343–56. - PMC - PubMed
- Abdullahi W, Tripathi D, Ronaldson PT. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. 2018;315(3):C343–56. - PMC - PubMed
-
- Schoknecht K, David Y, Heinemann U. The blood-brain barrier-gatekeeper to neuronal homeostasis: clinical implications in the setting of stroke. SemCell Developmental Biol. 2015;38:35–42. - PubMed
- Schoknecht K, David Y, Heinemann U. The blood-brain barrier-gatekeeper to neuronal homeostasis: clinical implications in the setting of stroke. SemCell Developmental Biol. 2015;38:35–42. - PubMed
-
- Shah K, Abbruscato T. The role of blood-brain barrier transporters in pathophysiology and pharmacotherapy of stroke. Curr Pharm Des. 2014;20(10):1510–22. - PubMed
- Shah K, Abbruscato T. The role of blood-brain barrier transporters in pathophysiology and pharmacotherapy of stroke. Curr Pharm Des. 2014;20(10):1510–22. - PubMed
-
- Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163–164:144–71. - PMC - PubMed
- Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J. et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163–164:144–71. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
