Comparison of IgA, IgG, and Neutralizing Antibody Responses Following Immunization With Moderna, BioNTech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm's COVID-19 Vaccines
- PMID: 35799790
- PMCID: PMC9254618
- DOI: 10.3389/fimmu.2022.917905
Comparison of IgA, IgG, and Neutralizing Antibody Responses Following Immunization With Moderna, BioNTech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm's COVID-19 Vaccines
Abstract
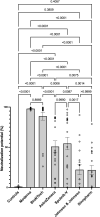
In an ongoing multinational trial, we obtained blood samples from 365 volunteers vaccinated with mRNA vaccines (Moderna, BioNTech), viral DNA-vectored vaccines (AstraZeneca, Sputnik-V, and Johnson and Johnson), or the attenuated virus vaccine from Sinopharm. After collecting reactogenicity data, the expression of S-Protein binding IgG and IgA was analyzed using an automated sandwich ELISA system. Serum neutralizing potentials were then investigated using an ACE-2-RBD neutralizing assay. Moderna's vaccine induced the highest amounts of SARS-CoV-2 specific neutralizing antibodies compared to the other groups. In contrast, Sinopharm and Johnson and Johnson's vaccinees presented the lowest SARS-CoV-2-specific antibody titers. Interestingly, moderate to high negative correlations between age and virus-specific IgG expression were observed in the Johnson and Johnson (ρ =-0.3936) and Sinopharm (ρ =-0.6977) groups according to Spearman's rank correlation analysis. A negative correlation was seen between age and IgA expression in the Sputnik-V group (ρ =-0.3917). The analysis of virus neutralization potentials in age categories demonstrated that no significant neutralization potential was observed in older vaccinees (61and 80 years old) in the Sputnik-V Johnson and Johnson and Sinopharm vaccinees' groups. In contrast, neutralization potentials in sera of Moderna, BioNTech, and AstraZeneca vaccinees were statistically comparable in all age categories. Furthermore, while the AstraZeneca vaccine alone induced moderate IgG and IgA expression, the combination with Moderna or BioNTech mRNA vaccines induced significantly higher antibody levels than a double dose of AstraZeneca and similar IgG expression and neutralization potential compared to Moderna or BioNTech vaccines used alone. These results suggest that mRNA vaccines are the most immunogenic after two doses. DNA vectored vaccines from AstraZeneca and Sputnik-V presented lower but significant antibody expression and virus neutralizing properties after two doses. The lowest antibody and neutralization potential were observed in the Sinopharm or Johnson and Johnson vaccinees. Especially elderly over 60 presented no significant increase in neutralizing antibodies after vaccination. The data also indicate that heterologous vaccination strategies combining the AstraZeneca DNA vectored vaccines and mRNA vaccines are more effective in the induction of neutralizing antibodies compared to their homologous counterparts.
Keywords: COVID-19 vaccines; ELISA; IgA; IgG; SARS-CoV-2; neutralizing antibodies.
Copyright © 2022 Adjobimey, Meyer, Sollberg, Bawolt, Berens, Kovačević, Trudić, Parcina and Hoerauf.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Comparison of the effectiveness and duration of anti-RBD SARS-CoV-2 IgG antibody response between different types of vaccines: Implications for vaccine strategies.Vaccine. 2022 May 3;40(20):2841-2847. doi: 10.1016/j.vaccine.2022.03.069. Epub 2022 Apr 1. Vaccine. 2022. PMID: 35397946 Free PMC article.
-
Adverse events and SARS-CoV-2 antibody responses after immunization with Sputnik V, ChAdOx1-S, and BBIBP-CorV vaccines in people with HIV.AIDS. 2023 May 1;37(6):941-946. doi: 10.1097/QAD.0000000000003483. Epub 2023 Jan 13. AIDS. 2023. PMID: 36728228 Free PMC article.
-
Assessing the long-stand antibody response induced by COVID-19 vaccines: A study in an educational cohort in San Luis, Argentina.Vaccine. 2023 Jan 9;41(2):476-485. doi: 10.1016/j.vaccine.2022.11.019. Epub 2022 Nov 21. Vaccine. 2023. PMID: 36481109 Free PMC article.
-
Saliva is suitable for SARS-CoV-2 antibodies detection after vaccination: A rapid systematic review.Front Immunol. 2022 Sep 20;13:1006040. doi: 10.3389/fimmu.2022.1006040. eCollection 2022. Front Immunol. 2022. PMID: 36203571 Free PMC article.
-
Immunotherapy for COVID-19: Evolving treatment of viral infection and associated adverse immunological reactions.Transfus Apher Sci. 2021 Apr;60(2):103093. doi: 10.1016/j.transci.2021.103093. Epub 2021 Feb 13. Transfus Apher Sci. 2021. PMID: 33610448 Free PMC article. Review.
Cited by
-
Role of Immunoglobulin A in COVID-19 and Influenza Infections.Vaccines (Basel). 2023 Oct 27;11(11):1647. doi: 10.3390/vaccines11111647. Vaccines (Basel). 2023. PMID: 38005979 Free PMC article.
-
Vaccine-induced humoral response of BNT162b2 and mRNA-1273 against BA.1, BA.5, and XBB.1.5. (sub)variants 6 months after a homologous booster: is immunogenicity equivalent?Heliyon. 2024 Aug 10;10(16):e36116. doi: 10.1016/j.heliyon.2024.e36116. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39247272 Free PMC article.
-
Comparative Analysis of SARS-CoV-2 Antibody Responses across Global and Lesser-Studied Vaccines.Vaccines (Basel). 2024 Mar 19;12(3):326. doi: 10.3390/vaccines12030326. Vaccines (Basel). 2024. PMID: 38543960 Free PMC article.
-
Evaluation of Anti-S1 IgA Response to Different COVID-19 Vaccination Regimens.Vaccines (Basel). 2023 Jun 19;11(6):1117. doi: 10.3390/vaccines11061117. Vaccines (Basel). 2023. PMID: 37376506 Free PMC article.
-
Longitudinal analysis of SARS-CoV-2 IgG antibody durability in Puerto Rico.Sci Rep. 2024 Dec 28;14(1):30743. doi: 10.1038/s41598-024-80465-4. Sci Rep. 2024. PMID: 39730470 Free PMC article.
References
-
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. . Safety and Immunogenicity of Chadox1 Ncov-19 Vaccine Administered in a Prime-Boost Regimen in Young and Old Adults (Cov002): A Single-Blind, Randomised, Controlled, Phase 2/3 Trial. Lancet (2021) 396(10267):1979–93. doi: 10.1016/S0140-6736(20)32466-1 - DOI - PMC - PubMed
-
- WHO . Coronavirus Disease (Covid-19) Dashboard (2021) (2021). Available at: https://covid19.who.int/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

