RhodiumIII-catalyzed remote difunctionalization of arenes assisted by a relay directing group
- PMID: 35799802
- PMCID: PMC9214915
- DOI: 10.1039/d2sc02205b
RhodiumIII-catalyzed remote difunctionalization of arenes assisted by a relay directing group
Abstract
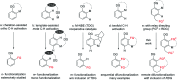
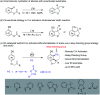
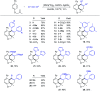
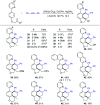
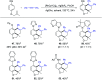
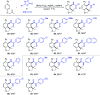
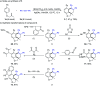
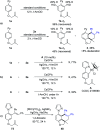
Rhodium-catalyzed diverse tandem twofold C-H bond activation reactions of para-olefin-tethered arenes have been realized, with unsaturated reagents such as internal alkynes, dioxazolones, and isocyanates being the coupling partner as well as a relay directing group which triggers cyclization of the para-olefin group under oxidative or redox-neutral conditions. The reaction proceeded via initial ortho-C-H activation assisted by a built-in directing group in the arene, and the ortho-incorporation of the unsaturated coupling partner simultaneously generated a relay directing group that allows sequential C-H activation at the meta-position and subsequent cyclization of the para-olefins. The overall reaction represents C-C or N-C difunctionalization of the arene with the generation of diverse 2,3-dihydrobenzofuran platforms. The catalytic system proceeded with good efficiency, simple reaction conditions, and broad substrate scope. The diverse transformations of the products demonstrated the synthetic utility of this tandem reaction.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Rhodium-catalyzed atroposelective access to trisubstituted olefins via C-H bond olefination of diverse arenes.Chem Sci. 2023 Jul 3;14(29):7999-8005. doi: 10.1039/d3sc02714g. eCollection 2023 Jul 26. Chem Sci. 2023. PMID: 37502336 Free PMC article.
-
Substrate activation strategies in rhodium(III)-catalyzed selective functionalization of arenes.Acc Chem Res. 2015 Apr 21;48(4):1007-20. doi: 10.1021/acs.accounts.5b00077. Epub 2015 Apr 6. Acc Chem Res. 2015. PMID: 25844661
-
Advances in Rhodium-Catalyzed Oxidative Arene Alkenylation.Acc Chem Res. 2020 Apr 21;53(4):920-936. doi: 10.1021/acs.accounts.0c00036. Epub 2020 Apr 2. Acc Chem Res. 2020. PMID: 32239913
-
Recent Advancements in Transition-Metal-Catalyzed One-Pot Twofold Unsymmetrical Difunctionalization of Arenes.Chem Asian J. 2019 Dec 2;14(23):4074-4086. doi: 10.1002/asia.201901213. Epub 2019 Oct 23. Chem Asian J. 2019. PMID: 31584753 Review.
-
Ruthenium-catalyzed hydroarylation reactions as the strategy towards the synthesis of alkylated arenes and substituted alkenes.RSC Adv. 2023 Feb 21;13(9):6246-6263. doi: 10.1039/d3ra00211j. eCollection 2023 Feb 14. RSC Adv. 2023. PMID: 36825293 Free PMC article. Review.
Cited by
-
Ruthenium-catalysed decarboxylative unsymmetric dual ortho-/meta-C-H bond functionalization of arenecarboxylic acids.Chem Sci. 2023 Apr 24;14(20):5470-5476. doi: 10.1039/d3sc01226c. eCollection 2023 May 24. Chem Sci. 2023. PMID: 37234909 Free PMC article.
-
Development of novel transition metal-catalyzed synthetic approaches for the synthesis of a dihydrobenzofuran nucleus: a review.RSC Adv. 2024 May 3;14(21):14539-14581. doi: 10.1039/d4ra01830c. eCollection 2024 May 2. RSC Adv. 2024. PMID: 38708111 Free PMC article. Review.
References
-
-
For selected reviews of transition-metal-catalyzed C–H functionalization, see:
- McMurray L. O'Hara F. Gaunt M. J. Chem. Soc. Rev. 2011;40:1885–1898. doi: 10.1039/C1CS15013H. - DOI - PubMed
- Cho S. H. Kim J. Y. Kwak J. Chang S. Chem. Soc. Rev. 2011;40:5068–5083. doi: 10.1039/C1CS15082K. - DOI - PubMed
- Ackermann L. Chem. Rev. 2011;111:1315–1345. doi: 10.1021/cr100412j. - DOI - PubMed
- Li B.-J. Shi Z.-J. Chem. Soc. Rev. 2012;41:5588–5598. doi: 10.1039/C2CS35096C. - DOI - PubMed
- Arockiam P. B. Bruneau C. Dixneuf P. H. Chem. Rev. 2012;112:5879–5918. doi: 10.1021/cr300153j. - DOI - PubMed
- Shi Z. Koester D. C. Boultadakis-Arapinis M. Glorius F. J. Am. Chem. Soc. 2013;135:12204–12207. doi: 10.1021/ja406338r. - DOI - PubMed
- Chen Z. Wang B. Zhang J. Yu W. Liu Z. Zhang Y. Org. Chem. Front. 2015;2:1107–1295. doi: 10.1039/C5QO00004A. - DOI
- Song G. Li X. Acc. Chem. Res. 2015;48:1007–1020. doi: 10.1021/acs.accounts.5b00077. - DOI - PubMed
- Gensch T. Hopkinson M. N. Glorius F. Wencel-Delord J. Chem. Soc. Rev. 2016;45:2900–2936. doi: 10.1039/C6CS00075D. - DOI - PubMed
- Cera G. Ackermann L. Top. Curr. Chem. 2016;374:191. - PubMed
- Yang Y. Lan J. You J. Chem. Rev. 2017;117:8787–8863. doi: 10.1021/acs.chemrev.6b00567. - DOI - PubMed
- Hummel J. R. Boerth J. A. Ellman J. A. Chem. Rev. 2017;117:9163–9227. doi: 10.1021/acs.chemrev.6b00661. - DOI - PMC - PubMed
- Sambiagio C. Schönbauer D. Blieck R. Dao-Huy T. Pototschnig G. Schaaf P. Wiesinger T. Zia M. F. Wencel-Delord J. Besset T. Maes B. U. W. Schnürch M. Chem. Soc. Rev. 2018;47:6603–6743. doi: 10.1039/C8CS00201K. - DOI - PMC - PubMed
- Gandeepan P. Müller T. Zell D. Cera G. Warratz S. Ackermann L. Chem. Rev. 2019;119:2192–2452. doi: 10.1021/acs.chemrev.8b00507. - DOI - PubMed
- Dutta U. Maiti S. Bhattacharya T. Maiti D. Science. 2021;372 doi: 10.1126/science.abd5992. doi: 10.1126/science.abd5992. - DOI - DOI - PubMed
- Yu X. Zhang Z.-Z. Niu J.-L. Shi B.-F. Org. Chem. Front. 2022;9:1458–1484. doi: 10.1039/D1QO01884A. - DOI
-
-
-
For selected examples, see:
- Ueura K. Satoh T. Miura M. Org. Lett. 2007;9:1407–1409. doi: 10.1021/ol070406h. - DOI - PubMed
- Ueura K. Satoh T. Miura M. J. Org. Chem. 2007;72:5362–5367. doi: 10.1021/jo070735n. - DOI - PubMed
- Rakshit S. Grohmann C. Besset T. Glorius F. J. Am. Chem. Soc. 2011;133:2350–2353. doi: 10.1021/ja109676d. - DOI - PubMed
- Guimond N. Gorelsky S. I. Fagnou K. J. Am. Chem. Soc. 2011;133:6449–6457. doi: 10.1021/ja201143v. - DOI - PubMed
- Hyster T. K. Knörr L. Ward T. R. Rovis T. Science. 2012;338:500–503. doi: 10.1126/science.1226132. - DOI - PMC - PubMed
- Hyster T. K. Dalton D. M. Rovis T. Chem. Sci. 2015;6:254–258. doi: 10.1039/C4SC02590C. - DOI - PMC - PubMed
- Semakul N. Jackson K. E. Paton R. S. Rovis T. Chem. Sci. 2017;8:1015–1020. doi: 10.1039/C6SC02587K. - DOI - PMC - PubMed
- Piou T. Romanov-Michailidis F. Romanova-Michaelides M. Jackson K. E. Semakul N. Taggart T. D. Newell B. S. Rithner C. D. Paton R. S. Rovis T. J. Am. Chem. Soc. 2017;139:1296–1310. doi: 10.1021/jacs.6b11670. - DOI - PMC - PubMed
- Ye B. Cramer N. Science. 2012;338:504–506. doi: 10.1126/science.1226938. - DOI - PubMed
- Wodrich M. D. Ye B. Gonthier J. F. Corminboeuf C. Cramer N. Chem.–Eur. J. 2014;20:15409–15418. doi: 10.1002/chem.201404515. - DOI - PubMed
- Wang D. Wang F. Song G. Li X. Angew. Chem., Int. Ed. 2012;51:12348–12352. doi: 10.1002/anie.201206918. - DOI - PubMed
- Qi Z. Wang M. Li X. Chem. Commun. 2014;50:9776–9778. doi: 10.1039/C4CC03627A. - DOI - PubMed
- Nguyen T. T. Grigorjeva L. Daugulis O. Angew. Chem., Int. Ed. 2018;57:1688–1691. doi: 10.1002/anie.201711968. - DOI - PMC - PubMed
-
-
- Lee S. Lee H. Tan K. L. J. Am. Chem. Soc. 2013;135:18778–18781. doi: 10.1021/ja4107034. - DOI - PMC - PubMed
- Tang R.-Y. Li G. Yu J.-Q. Nature. 2014;507:215–220. doi: 10.1038/nature12963. - DOI - PMC - PubMed
- Yang G. Lindovska P. Zhu D. Kim J. Wang P. Tang R.-Y. Movassaghi M. Yu J.-Q. J. Am. Chem. Soc. 2014;136:10807–10813. doi: 10.1021/ja505737x. - DOI - PubMed
- Bera M. Modak A. Patra T. Maji A. Maiti D. Org. Lett. 2014;16:5760–5763. doi: 10.1021/ol502823c. - DOI - PubMed
- Bera M. Maji A. Sahoo S. K. Maiti D. Angew. Chem., Int. Ed. 2015;54:8515–8519. doi: 10.1002/anie.201503112. - DOI - PubMed
- Chu L. Shang M. Tanaka K. Chen Q. Pissarnitski N. Streckfuss E. Yu J.-Q. ACS Cent. Sci. 2015;1:394–399. doi: 10.1021/acscentsci.5b00312. - DOI - PMC - PubMed
- Patra T. Watile R. Agasti S. Naveen T. Maiti D. Chem. Commun. 2016;52:2027–2030. doi: 10.1039/C5CC09446A. - DOI - PubMed
- Li S. Cai L. Ji H. Yang L. Li G. Nat. Commun. 2016;7:10443–10450. doi: 10.1038/ncomms10443. - DOI - PMC - PubMed
- Maji A. Bhaskararao B. Singha S. Sunoj R. B. Maiti D. Chem. Sci. 2016;7:3147–3153. doi: 10.1039/C5SC04060D. - DOI - PMC - PubMed
- Bera M. Sahoo S. K. Maiti D. ACS Catal. 2016;6:3575–3579. doi: 10.1021/acscatal.6b00675. - DOI
- Xu H.-J. Lu Y. Farmer M. E. Wang H.-W. Zhao D. Kang Y.-S. Sun W.-Y. Yu J.-Q. J. Am. Chem. Soc. 2017;139:2200–2203. doi: 10.1021/jacs.6b13269. - DOI - PubMed
- Bag S. Jayarajan R. Mondal R. Maiti D. Angew. Chem., Int. Ed. 2017;56:3182–3186. doi: 10.1002/anie.201611360. - DOI - PubMed
- Bera M. Agasti S. Chowdhury R. Mondal R. Pal D. Maiti D. Angew. Chem., Int. Ed. 2017;56:5272–5276. doi: 10.1002/anie.201701579. - DOI - PubMed
- Dutta U. Modak A. Bhaskararao B. Bera M. Bag S. Mondal A. Lupton D. W. Sunoj R. B. Maiti D. ACS Catal. 2017;7:3162–3168. doi: 10.1021/acscatal.7b00247. - DOI
- Fang L. Saint-Denis T. G. Taylor B. L. H. Ahlquist S. Hong K. Liu S. S. Han L. L. Houk K. N. Yu J.-Q. J. Am. Chem. Soc. 2017;139:10702–10714. doi: 10.1021/jacs.7b03296. - DOI - PMC - PubMed
- Xu H.-J. Kang Y.-S. Shi H. Zhang P. Chen Y.-K. Zhang B. Liu Z.-Q. Zhao J. Sun W.-Y. Yu J.-Q. Lu Y. J. Am. Chem. Soc. 2019;141:76–79. doi: 10.1021/jacs.8b11038. - DOI - PubMed
- Achar T. K. Zhang X. Mondal R. Shanavas M. S. Maiti S. Maity S. Pal N. Paton R. S. Maiti D. Angew. Chem., Int. Ed. 2019;58:10353–10360. doi: 10.1002/anie.201904608. - DOI - PubMed
- Li S. Wang H. Weng Y. Li G. Angew. Chem., Int. Ed. 2019;58:18502–18507. doi: 10.1002/anie.201910691. - DOI - PubMed
- Porey S. Zhang X. Bhowmick S. Singh V. K. Guin S. Paton R. S. Maiti D. J. Am. Chem. Soc. 2020;142:3762–3774. doi: 10.1021/jacs.9b10646. - DOI - PubMed
- Bag S. S. K. Mondal A. Jayarajan R. Dutta U. Porey S. Sunoj R. B. Maiti D. J. Am. Chem. Soc. 2020;142:12453–12466. doi: 10.1021/jacs.0c05223. - DOI - PubMed
- Bag S. Jana S. Pradhan S. Bhowmick S. Goswami N. Sinha S. K. Maiti D. Nat. Commun. 2021;12:1393. doi: 10.1038/s41467-021-21633-2. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

