RhodiumIII-catalyzed remote difunctionalization of arenes assisted by a relay directing group
- PMID: 35799802
- PMCID: PMC9214915
- DOI: 10.1039/d2sc02205b
RhodiumIII-catalyzed remote difunctionalization of arenes assisted by a relay directing group
Abstract
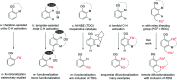
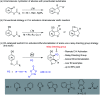
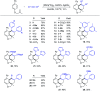
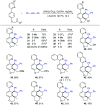
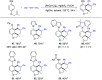
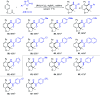
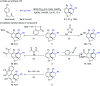
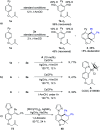
Rhodium-catalyzed diverse tandem twofold C-H bond activation reactions of para-olefin-tethered arenes have been realized, with unsaturated reagents such as internal alkynes, dioxazolones, and isocyanates being the coupling partner as well as a relay directing group which triggers cyclization of the para-olefin group under oxidative or redox-neutral conditions. The reaction proceeded via initial ortho-C-H activation assisted by a built-in directing group in the arene, and the ortho-incorporation of the unsaturated coupling partner simultaneously generated a relay directing group that allows sequential C-H activation at the meta-position and subsequent cyclization of the para-olefins. The overall reaction represents C-C or N-C difunctionalization of the arene with the generation of diverse 2,3-dihydrobenzofuran platforms. The catalytic system proceeded with good efficiency, simple reaction conditions, and broad substrate scope. The diverse transformations of the products demonstrated the synthetic utility of this tandem reaction.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
-
For selected reviews of transition-metal-catalyzed C–H functionalization, see:
- McMurray L. O'Hara F. Gaunt M. J. Chem. Soc. Rev. 2011;40:1885–1898. doi: 10.1039/C1CS15013H. - DOI - PubMed
- Cho S. H. Kim J. Y. Kwak J. Chang S. Chem. Soc. Rev. 2011;40:5068–5083. doi: 10.1039/C1CS15082K. - DOI - PubMed
- Ackermann L. Chem. Rev. 2011;111:1315–1345. doi: 10.1021/cr100412j. - DOI - PubMed
- Li B.-J. Shi Z.-J. Chem. Soc. Rev. 2012;41:5588–5598. doi: 10.1039/C2CS35096C. - DOI - PubMed
- Arockiam P. B. Bruneau C. Dixneuf P. H. Chem. Rev. 2012;112:5879–5918. doi: 10.1021/cr300153j. - DOI - PubMed
- Shi Z. Koester D. C. Boultadakis-Arapinis M. Glorius F. J. Am. Chem. Soc. 2013;135:12204–12207. doi: 10.1021/ja406338r. - DOI - PubMed
- Chen Z. Wang B. Zhang J. Yu W. Liu Z. Zhang Y. Org. Chem. Front. 2015;2:1107–1295. doi: 10.1039/C5QO00004A. - DOI
- Song G. Li X. Acc. Chem. Res. 2015;48:1007–1020. doi: 10.1021/acs.accounts.5b00077. - DOI - PubMed
- Gensch T. Hopkinson M. N. Glorius F. Wencel-Delord J. Chem. Soc. Rev. 2016;45:2900–2936. doi: 10.1039/C6CS00075D. - DOI - PubMed
- Cera G. Ackermann L. Top. Curr. Chem. 2016;374:191. - PubMed
- Yang Y. Lan J. You J. Chem. Rev. 2017;117:8787–8863. doi: 10.1021/acs.chemrev.6b00567. - DOI - PubMed
- Hummel J. R. Boerth J. A. Ellman J. A. Chem. Rev. 2017;117:9163–9227. doi: 10.1021/acs.chemrev.6b00661. - DOI - PMC - PubMed
- Sambiagio C. Schönbauer D. Blieck R. Dao-Huy T. Pototschnig G. Schaaf P. Wiesinger T. Zia M. F. Wencel-Delord J. Besset T. Maes B. U. W. Schnürch M. Chem. Soc. Rev. 2018;47:6603–6743. doi: 10.1039/C8CS00201K. - DOI - PMC - PubMed
- Gandeepan P. Müller T. Zell D. Cera G. Warratz S. Ackermann L. Chem. Rev. 2019;119:2192–2452. doi: 10.1021/acs.chemrev.8b00507. - DOI - PubMed
- Dutta U. Maiti S. Bhattacharya T. Maiti D. Science. 2021;372 doi: 10.1126/science.abd5992. doi: 10.1126/science.abd5992. - DOI - PubMed
- Yu X. Zhang Z.-Z. Niu J.-L. Shi B.-F. Org. Chem. Front. 2022;9:1458–1484. doi: 10.1039/D1QO01884A. - DOI
-
-
-
For selected examples, see:
- Ueura K. Satoh T. Miura M. Org. Lett. 2007;9:1407–1409. doi: 10.1021/ol070406h. - DOI - PubMed
- Ueura K. Satoh T. Miura M. J. Org. Chem. 2007;72:5362–5367. doi: 10.1021/jo070735n. - DOI - PubMed
- Rakshit S. Grohmann C. Besset T. Glorius F. J. Am. Chem. Soc. 2011;133:2350–2353. doi: 10.1021/ja109676d. - DOI - PubMed
- Guimond N. Gorelsky S. I. Fagnou K. J. Am. Chem. Soc. 2011;133:6449–6457. doi: 10.1021/ja201143v. - DOI - PubMed
- Hyster T. K. Knörr L. Ward T. R. Rovis T. Science. 2012;338:500–503. doi: 10.1126/science.1226132. - DOI - PMC - PubMed
- Hyster T. K. Dalton D. M. Rovis T. Chem. Sci. 2015;6:254–258. doi: 10.1039/C4SC02590C. - DOI - PMC - PubMed
- Semakul N. Jackson K. E. Paton R. S. Rovis T. Chem. Sci. 2017;8:1015–1020. doi: 10.1039/C6SC02587K. - DOI - PMC - PubMed
- Piou T. Romanov-Michailidis F. Romanova-Michaelides M. Jackson K. E. Semakul N. Taggart T. D. Newell B. S. Rithner C. D. Paton R. S. Rovis T. J. Am. Chem. Soc. 2017;139:1296–1310. doi: 10.1021/jacs.6b11670. - DOI - PMC - PubMed
- Ye B. Cramer N. Science. 2012;338:504–506. doi: 10.1126/science.1226938. - DOI - PubMed
- Wodrich M. D. Ye B. Gonthier J. F. Corminboeuf C. Cramer N. Chem.–Eur. J. 2014;20:15409–15418. doi: 10.1002/chem.201404515. - DOI - PubMed
- Wang D. Wang F. Song G. Li X. Angew. Chem., Int. Ed. 2012;51:12348–12352. doi: 10.1002/anie.201206918. - DOI - PubMed
- Qi Z. Wang M. Li X. Chem. Commun. 2014;50:9776–9778. doi: 10.1039/C4CC03627A. - DOI - PubMed
- Nguyen T. T. Grigorjeva L. Daugulis O. Angew. Chem., Int. Ed. 2018;57:1688–1691. doi: 10.1002/anie.201711968. - DOI - PMC - PubMed
-
-
- Lee S. Lee H. Tan K. L. J. Am. Chem. Soc. 2013;135:18778–18781. doi: 10.1021/ja4107034. - DOI - PMC - PubMed
- Tang R.-Y. Li G. Yu J.-Q. Nature. 2014;507:215–220. doi: 10.1038/nature12963. - DOI - PMC - PubMed
- Yang G. Lindovska P. Zhu D. Kim J. Wang P. Tang R.-Y. Movassaghi M. Yu J.-Q. J. Am. Chem. Soc. 2014;136:10807–10813. doi: 10.1021/ja505737x. - DOI - PubMed
- Bera M. Modak A. Patra T. Maji A. Maiti D. Org. Lett. 2014;16:5760–5763. doi: 10.1021/ol502823c. - DOI - PubMed
- Bera M. Maji A. Sahoo S. K. Maiti D. Angew. Chem., Int. Ed. 2015;54:8515–8519. doi: 10.1002/anie.201503112. - DOI - PubMed
- Chu L. Shang M. Tanaka K. Chen Q. Pissarnitski N. Streckfuss E. Yu J.-Q. ACS Cent. Sci. 2015;1:394–399. doi: 10.1021/acscentsci.5b00312. - DOI - PMC - PubMed
- Patra T. Watile R. Agasti S. Naveen T. Maiti D. Chem. Commun. 2016;52:2027–2030. doi: 10.1039/C5CC09446A. - DOI - PubMed
- Li S. Cai L. Ji H. Yang L. Li G. Nat. Commun. 2016;7:10443–10450. doi: 10.1038/ncomms10443. - DOI - PMC - PubMed
- Maji A. Bhaskararao B. Singha S. Sunoj R. B. Maiti D. Chem. Sci. 2016;7:3147–3153. doi: 10.1039/C5SC04060D. - DOI - PMC - PubMed
- Bera M. Sahoo S. K. Maiti D. ACS Catal. 2016;6:3575–3579. doi: 10.1021/acscatal.6b00675. - DOI
- Xu H.-J. Lu Y. Farmer M. E. Wang H.-W. Zhao D. Kang Y.-S. Sun W.-Y. Yu J.-Q. J. Am. Chem. Soc. 2017;139:2200–2203. doi: 10.1021/jacs.6b13269. - DOI - PubMed
- Bag S. Jayarajan R. Mondal R. Maiti D. Angew. Chem., Int. Ed. 2017;56:3182–3186. doi: 10.1002/anie.201611360. - DOI - PubMed
- Bera M. Agasti S. Chowdhury R. Mondal R. Pal D. Maiti D. Angew. Chem., Int. Ed. 2017;56:5272–5276. doi: 10.1002/anie.201701579. - DOI - PubMed
- Dutta U. Modak A. Bhaskararao B. Bera M. Bag S. Mondal A. Lupton D. W. Sunoj R. B. Maiti D. ACS Catal. 2017;7:3162–3168. doi: 10.1021/acscatal.7b00247. - DOI
- Fang L. Saint-Denis T. G. Taylor B. L. H. Ahlquist S. Hong K. Liu S. S. Han L. L. Houk K. N. Yu J.-Q. J. Am. Chem. Soc. 2017;139:10702–10714. doi: 10.1021/jacs.7b03296. - DOI - PMC - PubMed
- Xu H.-J. Kang Y.-S. Shi H. Zhang P. Chen Y.-K. Zhang B. Liu Z.-Q. Zhao J. Sun W.-Y. Yu J.-Q. Lu Y. J. Am. Chem. Soc. 2019;141:76–79. doi: 10.1021/jacs.8b11038. - DOI - PubMed
- Achar T. K. Zhang X. Mondal R. Shanavas M. S. Maiti S. Maity S. Pal N. Paton R. S. Maiti D. Angew. Chem., Int. Ed. 2019;58:10353–10360. doi: 10.1002/anie.201904608. - DOI - PubMed
- Li S. Wang H. Weng Y. Li G. Angew. Chem., Int. Ed. 2019;58:18502–18507. doi: 10.1002/anie.201910691. - DOI - PubMed
- Porey S. Zhang X. Bhowmick S. Singh V. K. Guin S. Paton R. S. Maiti D. J. Am. Chem. Soc. 2020;142:3762–3774. doi: 10.1021/jacs.9b10646. - DOI - PubMed
- Bag S. S. K. Mondal A. Jayarajan R. Dutta U. Porey S. Sunoj R. B. Maiti D. J. Am. Chem. Soc. 2020;142:12453–12466. doi: 10.1021/jacs.0c05223. - DOI - PubMed
- Bag S. Jana S. Pradhan S. Bhowmick S. Goswami N. Sinha S. K. Maiti D. Nat. Commun. 2021;12:1393. doi: 10.1038/s41467-021-21633-2. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

