Controlled assembly of a bicyclic porphyrinoid and its 3-dimensional boron difluoride arrays
- PMID: 35799810
- PMCID: PMC9214847
- DOI: 10.1039/d2sc01635d
Controlled assembly of a bicyclic porphyrinoid and its 3-dimensional boron difluoride arrays
Abstract
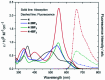
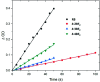
A fully conjugated cryptand-like bicyclic porphyrinoid ligand 4, incorporating three carbazole linkages and four dipyrrin moieties, was prepared via the acid-catalysed condensation of an extended 2,2'-bipyrrole analogue containing a central carbazole moiety and 3,4-diethyl-2,5-diformylpyrrole in 79% isolated yield. This new cryptand-like system acts as an effective ligand and allows for complexation of BF2 (boron difluoride) subunits. Three BODIPY arrays, containing two, three, and four BF2 subunits, namely 4·2BF2, 4·3BF2 and 4·4BF2, could thus be isolated from the reaction of 4 with BF3·Et2O in the presence of triethylamine at 110 °C, albeit in relatively low yield. As prepared, bicycle 4 is characterized by a rigid C 2 symmetric structure as inferred from VT NMR spectroscopic analyses. In contrast, the three BODIPY-like arrays produced as the result of BF2 complexation are conformationally flexible and unsymmetric in nature as deduced from similar analyses. All four products, namely 4, 4·2BF2, 4·3BF2 and 4·4BF2, were characterized by means of single crystal X-ray diffraction analyses. Tetramer 4·4BF2 gives rise to a higher extinction coefficient (by 2.5 times) relative to the bis- and tris-BODIPY arrays 4·2BF2 and 4·3BF2. This was taken as evidence for stronger excitonic coupling in the case of 4·4BF2. All three BODIPY-like arrays proved nearly non-fluorescent, as expected given their conformationally mobile nature. The efficiency of reactive oxygen species (ROS) generation was also determined for the new BODIPY arrays of this study.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

