Three-component carboacylation of alkenes via cooperative nickelaphotoredox catalysis
- PMID: 35799820
- PMCID: PMC9214884
- DOI: 10.1039/d2sc02277j
Three-component carboacylation of alkenes via cooperative nickelaphotoredox catalysis
Abstract
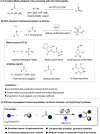
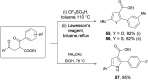
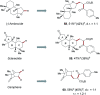
Various commercially available acyl chlorides, aldehydes, and alkanes were exploited for versatile three-component 1,2-carboacylations of alkenes to forge two vicinal C-C bonds through the cooperative action of nickel and sodium decatungstate catalysis. A wealth of ketones with high levels of structural complexity was rapidly obtained via direct functionalization of C(sp2)/C(sp3)-H bonds in a modular manner. Furthermore, a regioselective late-stage modification of natural products showcased the practical utility of the strategy, generally featuring high resource economy and ample substrate scope.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Derosa J. Apolinar O. Kang T. Tran V. T. Engle K. M. Chem. Sci. 2020;11:4287–4296. doi: 10.1039/C9SC06006E. - DOI - PMC - PubMed
- Zhang Z. Roisnel T. Dixneuf P. H. Soulé J.-F. Angew. Chem., Int. Ed. 2020;58:14110–14114. doi: 10.1002/anie.201906913. - DOI - PubMed
- Dhungana R. K. Kc S. Basnet P. Giri R. Chem. Rec. 2018;18:1314–1340. doi: 10.1002/tcr.201700098. - DOI - PubMed
- Zhang J. S. Liu L. Chen T. Han L. B. Chem.–Asian J. 2018;13:2277–2291. doi: 10.1002/asia.201800647. - DOI - PubMed
-
- Tu H. Wang F. Huo L. Li Y. Zhu S. Zhao X. Li H. Qing F. Chu L. J. Am. Chem. Soc. 2020;142:9604–9611. doi: 10.1021/jacs.0c03708. - DOI - PubMed
- Song L. Fu D.-M. Chen L. Jiang Y.-X. Ye J.-H. Zhu L. Lan Y. Fu Q. Yu D.-G. Angew. Chem., Int. Ed. 2020;59:21121–21128. doi: 10.1002/anie.202008630. - DOI - PubMed
- Wu X. Zhu C. Acc. Chem. Res. 2020;53:1620–1636. doi: 10.1021/acs.accounts.0c00306. - DOI - PubMed
- Li Z. Fang G. Gu Q. Liu X. Chem. Soc. Rev. 2020;49:32–48. doi: 10.1039/C9CS00681H. - DOI - PubMed
- Fan P. Lan Y. Zhang C. Wang C. J. Am. Chem. Soc. 2020;142:2180–2186. doi: 10.1021/jacs.9b12554. - DOI - PubMed
- Jiang H. Studer A. Chem. Soc. Rev. 2020;49:1790–1811. doi: 10.1039/C9CS00692C. - DOI - PubMed
- Li Z. Wang M. Shi Z. Angew. Chem., Int. Ed. 2020;60:186–190. doi: 10.1002/anie.202010839. - DOI - PubMed
- Wang X. Lu X. He S. Fu Y. Chem. Sci. 2020;11:7950–7956. doi: 10.1039/D0SC02054K. - DOI - PMC - PubMed
- Anthony D. Lin Q. Baudet J. Diao T. Angew. Chem., Int. Ed. 2019;58:3198–3202. doi: 10.1002/anie.201900228. - DOI - PMC - PubMed
- Wang D. Dong B. Wang Y. Qian J. Zhu J. Zhao Y. Shi Z. Nat. Commun. 2019;10:3539–3548. doi: 10.1038/s41467-019-11420-5. - DOI - PMC - PubMed
- Fu Q. Bo Z.-Y. Ye J.-H. Ju T. Huang H. Liao L.-L. Yu D.-G. Nat. Commun. 2019;10:3592–3600. doi: 10.1038/s41467-019-11528-8. - DOI - PMC - PubMed
- Zhang M. Xie J. Zhu C. Nat. Commun. 2018;9:3517–3526. doi: 10.1038/s41467-018-06019-1. - DOI - PMC - PubMed
- Wang F. Chen P. Liu G. Acc. Chem. Res. 2018;51:2036–2046. doi: 10.1021/acs.accounts.8b00265. - DOI - PubMed
- Yan M. Lo J. C. Edwards J. T. Baran P. S. J. Am. Chem. Soc. 2016;138:12692–12714. doi: 10.1021/jacs.6b08856. - DOI - PMC - PubMed
-
- Xiong T. Zhang Q. Chem. Soc. Rev. 2021;50:8857–8873. doi: 10.1039/D1CS00208B. - DOI - PubMed
- Sharma S. Singh J. Sharmaa A. Adv. Synth. Catal. 2021;363:3146–3169. doi: 10.1002/adsc.202100205. - DOI
- Pitzer L. Schwarz J. L. Glorius F. Chem. Sci. 2019;10:8285–8291. doi: 10.1039/C9SC03359A. - DOI - PMC - PubMed
- Jin Y. Wang C. Chem. Sci. 2019;10:1780–1785. doi: 10.1039/C8SC04279A. - DOI - PMC - PubMed
- Zhang Z. Gong L. Zhou X.-Y. Yan S.-S. Li J. Yu D.-G. Acta Chim. Sin. 2019;77:783–793. doi: 10.6023/A19060208. - DOI
- Koike T. Akita M. Chem. 2018;4:409–437. doi: 10.1016/j.chempr.2017.11.004. - DOI
- Sauer G. S. Lin S. ACS Catal. 2018;8:5175–5187. doi: 10.1021/acscatal.8b01069. - DOI
- Wang K. Ding Z. Zhou Z. Kong W. J. Am. Chem. Soc. 2018;140:12364–12368. doi: 10.1021/jacs.8b08190. - DOI - PubMed
-
- Wei X. Shu W. García-Domínguez A. Merino E. Nevado C. J. Am. Chem. Soc. 2020;142:13515–13522. doi: 10.1021/jacs.0c05254. - DOI - PubMed
- Shu W. García-Domínguez A. Quirls M. T. Mondal R. Cordenas D. J. Nevado C. J. Am. Chem. Soc. 2019;141:13812–13821. doi: 10.1021/jacs.9b02973. - DOI - PubMed
- García-Domínguez A. Mondal R. Nevado C. Angew. Chem., Int. Ed. 2019;58:12286–12290. doi: 10.1002/anie.201906692. - DOI - PubMed
- García-Domínguez A. Li Z. Nevado C. J. Am. Chem. Soc. 2017;139:6835–6838. doi: 10.1021/jacs.7b03195. - DOI - PubMed
- Merino E. Nevado C. Chem. Soc. Rev. 2014;43:6598–6608. doi: 10.1039/C4CS00025K. - DOI - PMC - PubMed
-
- Campbell M. W. Yuan M. Polites V. C. Gutierrez O. Molander G. A. J. Am. Chem. Soc. 2021;143:3901–3910. doi: 10.1021/jacs.0c13077. - DOI - PMC - PubMed
- Lipp A. Badir S. O. Molander G. A. Angew. Chem., Int. Ed. 2021;60:1714–1726. doi: 10.1002/anie.202007668. - DOI - PMC - PubMed
- Badir S. O. Molander G. A. Chem. 2020;6:1327–1339. doi: 10.1016/j.chempr.2020.05.013. - DOI - PMC - PubMed
- Campbell M. W. Compton J. S. Kelly C. B. Molander G. A. J. Am. Chem. Soc. 2019;141:20069–20078. doi: 10.1021/jacs.9b08282. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

