CXCR4/CX43 Regulate Diabetic Neuropathic Pain via Intercellular Interactions between Activated Neurons and Dysfunctional Astrocytes during Late Phase of Diabetes in Rats and the Effects of Antioxidant N-Acetyl-L-Cysteine
- PMID: 35799894
- PMCID: PMC9256426
- DOI: 10.1155/2022/8547563
CXCR4/CX43 Regulate Diabetic Neuropathic Pain via Intercellular Interactions between Activated Neurons and Dysfunctional Astrocytes during Late Phase of Diabetes in Rats and the Effects of Antioxidant N-Acetyl-L-Cysteine
Abstract
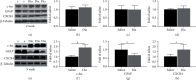
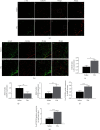
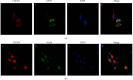
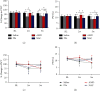
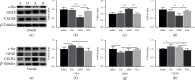
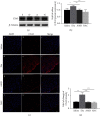
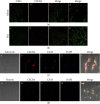
Growing evidence suggests that the interactions between astrocytes and neurons exert important functions in the central sensitization of the spinal cord dorsal horn in rodents with diabetes and neuropathic pain (DNP). However, it still remains unclear how signal transmission occurs in the spinal cord dorsal horn between astrocytes and neurons, especially in subjects with DNP. Chemokine CXC receptor 4 (CXCR4) plays critical roles in DNP, and connexin 43 (CX43), which is also primarily expressed by astrocytes, contributes to the development of neuropathy. We thus postulated that astrocytic and neuronal CXCR4 induces and produces inflammatory factors under persistent peripheral noxious stimulation in DNP, while intercellular CX43 can transmit inflammatory stimulation signals. The results showed that streptozotocin-induced type 1 diabetic rats developed heat hyperalgesia and mechanical allodynia. Diabetes led to persistent neuropathic pain. Diabetic rats developed peripheral sensitization at the early phase (2 weeks) and central sensitization at the late phase (5 weeks) after diabetes induction. Both CXCR4 and CX43, which are localized and coexpressed in neurons and astrocytes, were enhanced significantly in the dorsal horn of spinal cord in rats undergoing DNP during late phase of diabetes, and the CXCR4 antagonist AMD3100 reduced the expression of CX43. The nociceptive behavior was reversed, respectively, by AMD3100 at the early phase and by the antioxidant N-acetyl-L-cysteine (NAC) at the late phase. Furthermore, rats with DNP demonstrated downregulation of glial fibrillary acidic protein (GFAP) as well as upregulation of c-fos in the spinal cord dorsal horn at the late phase compared to the controls, and upregulation of GFAP and downregulation of c-fos were observed upon treatment with NAC. Given that GFAP and c-fos are, respectively, makers of astrocyte and neuronal activation, our findings suggest that CXCR4 as an inflammatory stimulation protein and CX43 as an intercellular signal transmission protein both may induce neurons excitability and astrocytes dysfunction in developing DNP.
Copyright © 2022 Dan Zhu et al.
Conflict of interest statement
This study is claimed to show no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

