Electrostatically optimized adapalene-loaded emulsion for the treatment of acne vulgaris
- PMID: 35799897
- PMCID: PMC9254491
- DOI: 10.1016/j.mtbio.2022.100339
Electrostatically optimized adapalene-loaded emulsion for the treatment of acne vulgaris
Abstract
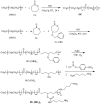
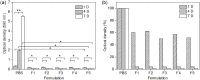
Adapalene (AD) is an FDA-approved drug that shows good therapeutic efficacy for the treatment of acne vulgaris. However, due to its negative charge, AD cannot efficiently penetrate across the also negatively-charged skin membrane. This study is the first to assess the treatment of acne vulgaris using electrostatically optimized AD emulsions prepared using anionic AD with methoxy polyethylene glycol-b-poly(ε-caprolactone) (MC) as an anionic emulsifier coupled with a newly synthesized MC with different contents of an amine pendant-group (MC-[NH2]x) as a cationic emulsifier. The AD emulsion prepared using MC-[NH2]x with high cationic charge potential was significantly stable in the short-term studies compared with anionic MC or no emulsifier. Furthermore, the AD emulsion prepared with the cationic MC-[NH2]x emulsifier provided a two or three times stronger therapeutic effect against acne vulgaris than the AD emulsion prepared with the anionic MC emulsifier or no emulsifier in an animal study. Additionally, the AD emulsion with high cationic charge potential exerted a remarkable inhibition of macrophage expression, as confirmed by histological analysis. Therefore, the electrostatic interaction between the negatively-charged skin membrane and the AD emulsion prepared with the cationic MC-[NH2]x emulsifier provides a promising therapeutic strategy for acne vulgaris.
Keywords: Acne vulgaris; Adapalene; Electrostatic emulsifier; Electrostatic interaction; Emulsion.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Tan H.H. Antibacterial therapy for acne: a guide to selection and use of systemic agents. Am. J. Clin. Dermatol. 2003;4(5):307–314. - PubMed
-
- Stuart B., Maund E., Wilcox C., Sridharan K., Sivaramakrishnan G., Regas C., Newell D., Soulsby I., Tang K.F., Finlay A.Y., Bucher H.C., Little P., Layton A.M., Santer M. Topical preparations for the treatment of mild-to-moderate acne vulgaris: systematic review and network meta-analysis. Br. J. Dermatol. 2021;185(3):512–525. - PubMed
-
- Aslam I., Fleischer A., Feldman S. Emerging drugs for the treatment of acne. Expet Opin. Emerg. Drugs. 2015;20(1):91–101. - PubMed
LinkOut - more resources
Full Text Sources

