Long-Term Safety and Efficacy of CD19 Humanized Selective CAR-T Therapy in B-ALL Patients Who Have Previously Received Murine-Based CD19 CAR-T Therapy
- PMID: 35800047
- PMCID: PMC9253302
- DOI: 10.3389/fonc.2022.884782
Long-Term Safety and Efficacy of CD19 Humanized Selective CAR-T Therapy in B-ALL Patients Who Have Previously Received Murine-Based CD19 CAR-T Therapy
Abstract
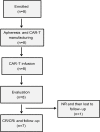
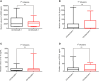
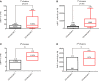
Murine-based CD19 CAR-T (CD19m CAR-T) therapy can lead to a relatively high CR rate when administered to B-ALL patients for the first time. However, the DOR is sub-optimal and a subset of patients even show primary resistance to CD19m CAR-T. To address these issues, we employed a humanized selective CD19CAR-T (CD19hs CAR-T) and evaluated the long-term safety and efficacy of treating 8 R/R B-ALL patients who had relapsed or failed to achieve CR following CD19m CAR-T infusion (Clinical trials' number: ChiCTR1800014761 and ChiCTR1800017439). Of the 8 patients, 7 achieved CR on Day 30 after the 1st infusion of CD19hs CAR-T. The median CRS grade was 1 without significant neurotoxicity seen in any of the 8 patients. The median DOR was 11 months, significantly longer than the DOR following CD19mCAR-T infusions. Anti-CAR antibodies were induced in patients who had received prior CD19m CAR-T infusions but not in those following a single or repeated CD19hsCAR-T treatment, which probably had contributed to the sub-optimal DOR and/or failure of effective response in these patients. CD19hs CAR-T, in contrast, induced low immunogenicity compared with CD19m CAR-T, suggesting that a repeat dosing strategy might be feasible and efficacious for patients who have relapsed and/or show primary resistance to CD19m CAR-T therapy. In this clinical study, CD19hs CAR-T showed a significant clinical efficacy with mild side effect among patients with R/R B-ALL who had previously received CD19m CAR-T.
Clinical trial registration: https://www.chictr.org.cn/showprojen.aspx?proj=25199 (ChiCTR1800014761). https://www.chictr.org.cn/showproj.aspx?proj=29174 (ChiCTR1800017439).
Keywords: B-ALL; CAR-T; HSCT; humanized; repeated dosing; selective domain.
Copyright © 2022 Zhao, Zhang, Yang, Wu, Chen, Li, Liu, Wang, Liu, Zhang, Zhou, Lu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

