Homologous recombination repair deficient prostate cancer represents an immunologically distinct subtype
- PMID: 35800157
- PMCID: PMC9255222
- DOI: 10.1080/2162402X.2022.2094133
Homologous recombination repair deficient prostate cancer represents an immunologically distinct subtype
Abstract
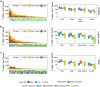
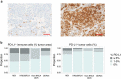
Homologous recombination repair deficiency (HRD) is observed in 10% of patients with castrate-resistant prostate cancer (PCa). Preliminary data suggest that HRD-PCa might be more responsive to immune checkpoint inhibitors (ICIs). In this study, we compare the tumor immune landscape and peripheral T cell receptor (TCR) repertoire of patients with and without HRD-PCa to gain further insight into the immunogenicity of HRD-PCa. Immunohistochemistry was performed on tumor tissue of 81 patients, including 15 patients with HRD-PCa. Peripheral TCR sequencing was performed in a partially overlapping cohort of 48 patients, including 16 patients with HRD-PCa. HRD patients more frequently had intratumoral CD3+, CD3+CD8-FoxP3- or Foxp3+ TILs above median compared to patients without DNA damage repair alterations (DDRwt; CD3+ and Foxp3+: 77% vs 35%, p = .013; CD3+CD8-FoxP3-: 80% vs 44%, p = .031). No significant difference in CD8+ TILs or PD-L1 expression was observed. In peripheral blood, HRD patients displayed a more diverse TCR repertoire compared to DDRwt patients (p = .014). Additionally, HRD patients shared TCR clusters with low generation probability, suggesting patient-overlapping T cell responses. A pooled analysis of clinical data from 227 patients with molecularly characterized PCa suggested increased efficacy of ICIs in HRD-PCa. In conclusion, patients with HRD-PCa display increased TIL density and an altered peripheral TCR repertoire. Further research into the efficacy of ICIs and the presence of shared neoantigens in HRD-PCa is warranted.
Keywords: Prostate cancer; homologous recombination repair deficiency; tumor-infiltrating lymphocytes.
© 2022 Radboud University Medical Center. Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
The authors report there are no competing interest to declare
Figures



References
-
- Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40–47. doi:10.1200/JCO.2016.69.1584 - DOI - PubMed
-
- Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, Krainer M, Houede N, Santos R, Mahammedi H, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi:10.1016/S1470-2045(14)70189-5 - DOI - PMC - PubMed
-
- Fizazi K, Drake CG, Beer TM, Kwon ED, Scher HI, Gerritsen WR, Bossi A, den Eertwegh AJMV, Krainer M, Houede N, et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase iii trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol. 2020;78(6):822–830. doi:10.1016/j.eururo.2020.07.032 - DOI - PMC - PubMed
-
- Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, Vaishampayan U, Berger R, Sezer A, Alanko T, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38(5):395–405. doi:10.1200/JCO.19.01638 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
