Occult Hepatitis B Infection among Hemodialysis in Tabriz, Northwest of Iran: Prevalence and Mutations within the S Region
- PMID: 35800327
- PMCID: PMC9256460
- DOI: 10.1155/2022/3838857
Occult Hepatitis B Infection among Hemodialysis in Tabriz, Northwest of Iran: Prevalence and Mutations within the S Region
Abstract
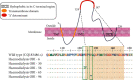
Regardless of the extensive screening for the detection of hepatitis B surface antigen (HBsAg), hemodialysis (HD) patients are still severely at the risk of occult hepatitis B virus infection (OBI), especially in developing countries. OBI is defined as the presence of HBV DNA with undetectable HBsAg in the liver and/or Serum. This study aims to determine the prevalence of OBI in HD patients in Tabriz Province, northwest of Iran, and inquire about the mutations in the detected HBsAg. In this cross-sectional descriptive study, ELISA method assessed serum and plasma samples of 118 HBsAg-negative patients undergoing HD treatment for HBV serological markers (HBsAg and Anti-HBc). Specific primers by nested polymerase chain reaction have been utilized to examine HBV DNA; also, direct sequencing of surface genes was carried out to characterize the viral genotypes and S gene mutations. Finally, followed by real-time PCR, the quantity of viral load in OBI-positive patients was determined. A total of 118 HD patients were included (63.6% were male and 36.4% female), with an overall mean age of 60.8 ± 12.8 years old. The prevalence of antihepatitis B core antibody (Anti-HBc) in the study population was 26.3% (31/118). Five patients (4.2%) were positive for HBV DNA and labeled OBI-positive; their plasma HBV-DNA load was less than 100 IU/ml. Following the phylogenetic analysis, the samples with OBI roughly belonged to genotype D, subtype ayw2 and only two had mutations within the S 'gene's major hydrophilic region (MHR), including T123I, C124F, and P127T. This study reports the prevalence of OBI in the HBsAg-negative HD patients being at a rate of 4.2%, which can be a clinically vital consideration in this region. HBV serologic screening approaches need to be renewed to cover nucleic acid testing in the setting of hemodialysis and all the other high-risk groups associated with it (i.e., blood and organ donors).
Copyright © 2022 Narges Eslami et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
High rate of occult hepatitis B virus infection in hemodialysis units of KwaZulu-Natal, South Africa.J Med Virol. 2019 Oct;91(10):1797-1803. doi: 10.1002/jmv.25510. Epub 2019 Jun 23. J Med Virol. 2019. PMID: 31180137
-
[Prevalence of viral hepatitis B markers among blood donors in the Republic of Guinea].Vopr Virusol. 2022 Mar 15;67(1):59-68. doi: 10.36233/0507-4088-92. Vopr Virusol. 2022. PMID: 35293189 Russian.
-
Molecular and Serological Characterization of Hepatitis B Virus (HBV)-Positive Samples with Very Low or Undetectable Levels of HBV Surface Antigen.Viruses. 2021 Oct 13;13(10):2053. doi: 10.3390/v13102053. Viruses. 2021. PMID: 34696483 Free PMC article.
-
Prevalence of occult hepatitis B virus infection in Egypt: a systematic review with meta-analysis.J Egypt Public Health Assoc. 2023 Jul 26;98(1):13. doi: 10.1186/s42506-023-00138-4. J Egypt Public Health Assoc. 2023. PMID: 37491501 Free PMC article. Review.
-
Occult hepatitis B virus infection: implications in transfusion.Vox Sang. 2004 Feb;86(2):83-91. doi: 10.1111/j.0042-9007.2004.00406.x. Vox Sang. 2004. PMID: 15023176 Review.
Cited by
-
Prevalence of Hepatitis B Virus, Hepatitis C Virus, and HIV Infections in Hemodialysis Patients at Kano Kidney Center.Cureus. 2023 Jul 12;15(7):e41769. doi: 10.7759/cureus.41769. eCollection 2023 Jul. Cureus. 2023. PMID: 37449288 Free PMC article.
-
Overt and occult hepatitis B virus infection detected among chronic kidney disease patients on haemodialysis at a Tertiary Hospital in Ghana.PLoS One. 2024 Mar 4;19(3):e0290917. doi: 10.1371/journal.pone.0290917. eCollection 2024. PLoS One. 2024. PMID: 38437229 Free PMC article.
-
M133S mutation possibly involve in the ER stress and mitophagy pathway in maintenance hemodialysis patients with occult hepatitis B infection.Sci Rep. 2024 Jun 17;14(1):13981. doi: 10.1038/s41598-024-64943-3. Sci Rep. 2024. PMID: 38886481 Free PMC article.
References
-
- World Health Organization. Hepatitis B Fact Sheet . Geneva, Switzerland: WHO; 2015.
LinkOut - more resources
Full Text Sources

