Venetoclax resistance: mechanistic insights and future strategies
- PMID: 35800373
- PMCID: PMC9255248
- DOI: 10.20517/cdr.2021.125
Venetoclax resistance: mechanistic insights and future strategies
Abstract
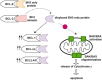
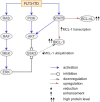
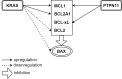
Acute myeloid leukemia (AML) is historically associated with poor prognosis, especially in older AML patients unfit for intensive chemotherapy. The development of Venetoclax, a potent oral BH3 (BCL-2 homology domain 3) mimetic, has transformed the AML treatment. However, the short duration of response and development of resistance remain major concerns. Understanding mechanisms of resistance is pivotal to devising new strategies and designing rational drug combination regimens. In this review, we will provide a comprehensive summary of the known mechanisms of resistance to Venetoclax and discuss Venetoclax-based combination therapies. Key contributing factors to Venetoclax resistance include dependencies on alternative anti-apoptotic BCL-2 family proteins and selection of the activating kinase mutations. Mutational landscape governing response to Venetoclax and strategic approaches developed considering current knowledge of mechanisms of resistance will be addressed.
Keywords: Azacitidine; BCL2 protein; Decitabine; Venetoclax; acute myeloid leukemia; human; hypomethylating agents; resistance.
© The Author(s) 2022.
Conflict of interest statement
Marina Konopleva: Consultant for AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, KisoJi, Janssen; serves as advisory board member for Stemline Therapeutics, F. Hoffman La-Roche, Janssen; holds shares from Reata Pharmaceuticals; honoraria from Forty-Seven and F. Hoffman La-Roche; research funding from AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Stemline Therapeutics, Ablynx, Agios, Ascentage, Astra Zeneca, Rafael Pharmaceutical, Sanofi, and Forty-Seven.
Figures
References
-
- Howlader N NA, Krapcho M, Miller D, et al. (eds) SEER Cancer Statistics Review, 1975-2017. National Cancer Institute. Available from: https://seer.cancer.gov/csr/1975_2017/ [Last accessed on 6 Apr 2022].
-
- Short NJ, Konopleva M, Kadia TM, et al. Advances in the treatment of acute myeloid leukemia: new drugs and new challenges. Cancer Discov. 2020;10:506–25. doi: 10.1158/2159-8290.CD-19-1011. - DOI - PubMed
-
- Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6:1106–17. doi: 10.1158/2159-8290.CD-16-0313. - DOI - PMC - PubMed
-
- Food and Drug Administration. FDA grants regular approval to venetoclax in combination for untreated acute myeloid leukemia. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grant.... [Last accessed on 6 Apr 2022]
Publication types
LinkOut - more resources
Full Text Sources
