Vaginal Microbiota Changes Caused by HPV Infection in Chinese Women
- PMID: 35800384
- PMCID: PMC9253274
- DOI: 10.3389/fcimb.2022.814668
Vaginal Microbiota Changes Caused by HPV Infection in Chinese Women
Abstract
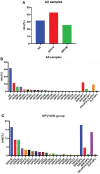
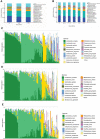
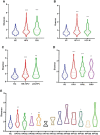
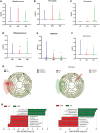
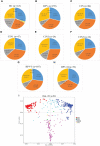
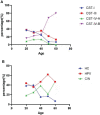
Human papillomavirus (HPV) infection is one of the most common sexually transmitted diseases. After studying 602 unvaccinated Chinese women using 16S rRNA to detect cervical-vaginal microecology, we analyzed the relationship between HPV infection and vaginal microecology including 20 HPV types. In Chinese women, L. gasseri-dominated and L. jensenii-dominated clusters were significantly absence. Microbial alpha diversity was significantly higher in HPV-infected and cervical intraepithelial neoplasia (CIN)-diagnosed groups than in healthy control group. Certain bacteria were associated with HPV infection and CIN, including Streptococcus, Prevotella, Chlamydia, Bifidobacterium, Ralstonia, and Aerococcus. With the development of disease, the proportions of community state type III (CST-III) and CST-IV-B gradually increased, whereas the proportions of CST-I and CST-IV-A gradually decreased. In addition, age was an influential factor for HPV infection. With aging, the probability of HPV infection and the proportion of CST-IV-B increase. In conclusion, our study was a large cross-sectional study that evaluated the relationship between vaginal microbiota and HPV infection, and brought essential comparable data.
Keywords: HPV infection; L. gasseri–dominated; age; cervical intraepithelial neoplasia; community state types (CST); vaginal microbiota.
Copyright © 2022 Zhang, Xu, Yu, Shi, Min, Xiong, Pan, Zhang, Liu, Wu and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort.BMC Infect Dis. 2020 Aug 26;20(1):629. doi: 10.1186/s12879-020-05324-9. BMC Infect Dis. 2020. PMID: 32842982 Free PMC article.
-
Human papillomavirus molecular prevalence in south China and the impact on vaginal microbiome of unvaccinated women.mSystems. 2024 Sep 17;9(9):e0073824. doi: 10.1128/msystems.00738-24. Epub 2024 Aug 9. mSystems. 2024. PMID: 39120153 Free PMC article.
-
The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: a cross-sectional analysis.BJOG. 2020 Jan;127(2):182-192. doi: 10.1111/1471-0528.15981. Epub 2019 Nov 20. BJOG. 2020. PMID: 31749298 Free PMC article.
-
The Vaginal Microbiota, Human Papillomavirus, and Cervical Dysplasia-A Review.Medicina (Kaunas). 2025 May 5;61(5):847. doi: 10.3390/medicina61050847. Medicina (Kaunas). 2025. PMID: 40428805 Free PMC article. Review.
-
Vaginal microecology and its role in human papillomavirus infection and human papillomavirus associated cervical lesions.APMIS. 2024 Dec;132(12):928-947. doi: 10.1111/apm.13356. Epub 2023 Nov 8. APMIS. 2024. PMID: 37941500 Review.
Cited by
-
Correlations between C-myc expression, BMI-1 expression, and vaginal microecology with HPV-DNA load in patients with different cervical lesions.Am J Transl Res. 2024 Jun 15;16(6):2544-2553. doi: 10.62347/GPLZ1377. eCollection 2024. Am J Transl Res. 2024. PMID: 39006286 Free PMC article.
-
Biomarkers differentiating regression from progression among untreated cervical intraepithelial neoplasia grade 2 lesions.J Adv Res. 2025 Aug;74:391-402. doi: 10.1016/j.jare.2024.09.009. Epub 2024 Sep 12. J Adv Res. 2025. PMID: 39260797 Free PMC article. Review.
-
Risk factors for hrHPV infection with cervical lesions patient in Anhui Province of China from 2021 to 2024: A retrospective study.Medicine (Baltimore). 2025 Jul 25;104(30):e43384. doi: 10.1097/MD.0000000000043384. Medicine (Baltimore). 2025. PMID: 40725893 Free PMC article.
-
Unique microbial diversity, community composition, and networks among Pacific Islander endocervical and vaginal microbiomes with and without Chlamydia trachomatis infection in Fiji.mBio. 2024 Jan 16;15(1):e0306323. doi: 10.1128/mbio.03063-23. Epub 2023 Dec 20. mBio. 2024. PMID: 38117091 Free PMC article.
-
ATP13A2 as a prognostic biomarker and its correlation with immune infiltration in cervical cancer: A retrospective study.J Cell Mol Med. 2025 Apr;29(7):e70097. doi: 10.1111/jcmm.70097. J Cell Mol Med. 2025. PMID: 40197818 Free PMC article.
References
-
- Borgdorff H., Gautam R., Armstrong S. D., Xia D., Ndayisaba G. F., van Teijlingen N. H., et al. . (2016). Cervicovaginal Microbiome Dysbiosis is Associated With Proteome Changes Related to Alterations of the Cervicovaginal Mucosal Barrier. Mucosal Immunol. 9 (3), 621–633. doi: 10.1038/mi.2015.86 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

