A Systematic Review of the Efficacy and Safety of Fecal Microbiota Transplantation in the Treatment of Hepatic Encephalopathy and Clostridioides difficile Infection in Patients With Cirrhosis
- PMID: 35800791
- PMCID: PMC9246246
- DOI: 10.7759/cureus.25537
A Systematic Review of the Efficacy and Safety of Fecal Microbiota Transplantation in the Treatment of Hepatic Encephalopathy and Clostridioides difficile Infection in Patients With Cirrhosis
Abstract
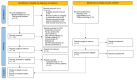
The microbiome of the human gut and liver coexists by influencing the health and disease state of each system. Fecal microbiota transplantation (FMT) has recently emerged as a potential treatment for conditions associated with cirrhosis, such as hepatic encephalopathy and recurrent/refractory Clostridioides difficile infection (rCDI). We have conducted a systematic review of the safety and efficacy of FMT in treating hepatic encephalopathy and rCDI. A literature search was performed using variations of the keywords "fecal microbiota transplant" and "cirrhosis" on PubMed/MEDLINE from inception to October 3, 2021. The resulting 116 articles were independently reviewed by two authors. Eight qualifying studies were included in the systematic review. A total of 127 cirrhotic patients received FMT. Hepatic encephalopathy was evaluated by cognitive tests, such as the Psychometric Hepatic Encephalopathy Score (PHES) and EncephalApp Stroop test. Not only was there an improvement in the cognitive performance in the FMT cohort, but the improvement was also maintained throughout long-term follow-up. In the treatment of rCDI, the FMT success rate is similar between cirrhotic patients and the general population, although more than one dose may be needed in the former. The rate of serious adverse events and adverse events in the cirrhotic cohort was slightly higher than that in the general population but was low overall. We found evidence that supports the therapeutic potential and safety profile of FMT to treat hepatic encephalopathy and rCDI in cirrhotic patients. Further research will be beneficial to better understand the role of FMT in cirrhosis.
Keywords: adverse outcomes; cirrhosis; clostridioides difficile infection; fecal microbiota transplantation; hepatic encephalopathy.
Copyright © 2022, Tun et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Fecal Microbiota Transplantation in Decompensated Cirrhosis: A Systematic Review on Safety and Efficacy.Antibiotics (Basel). 2022 Jun 23;11(7):838. doi: 10.3390/antibiotics11070838. Antibiotics (Basel). 2022. PMID: 35884093 Free PMC article. Review.
-
Microbiota restoration therapies for recurrent Clostridioides difficile infection reach an important new milestone.Therap Adv Gastroenterol. 2024 May 24;17:17562848241253089. doi: 10.1177/17562848241253089. eCollection 2024. Therap Adv Gastroenterol. 2024. PMID: 38800353 Free PMC article. Review.
-
Long-Term Efficacy and Safety of Fecal Microbiota Transplantation for Treatment of Recurrent Clostridioides difficile Infection.J Clin Gastroenterol. 2020 Sep;54(8):701-706. doi: 10.1097/MCG.0000000000001281. J Clin Gastroenterol. 2020. PMID: 32011405
-
Long-term durability and safety of fecal microbiota transplantation for recurrent or refractory Clostridioides difficile infection with or without antibiotic exposure.Eur J Clin Microbiol Infect Dis. 2019 Sep;38(9):1731-1735. doi: 10.1007/s10096-019-03602-2. Epub 2019 Jun 5. Eur J Clin Microbiol Infect Dis. 2019. PMID: 31165961
-
Safety and efficacy of fecal microbiota transplantation to treat and prevent recurrent Clostridioides difficile in cancer patients.J Cancer. 2021 Sep 7;12(21):6498-6506. doi: 10.7150/jca.59251. eCollection 2021. J Cancer. 2021. PMID: 34659541 Free PMC article.
Cited by
-
Role of fecal microbiota transplant in management of hepatic encephalopathy: Current trends and future directions.World J Hepatol. 2024 Jan 27;16(1):17-32. doi: 10.4254/wjh.v16.i1.17. World J Hepatol. 2024. PMID: 38313244 Free PMC article. Review.
-
Sarcopenia in cirrhosis: Prospects for therapy targeted to gut microbiota.World J Gastroenterol. 2023 Jul 21;29(27):4236-4251. doi: 10.3748/wjg.v29.i27.4236. World J Gastroenterol. 2023. PMID: 37545638 Free PMC article. Review.
-
Old and New Precipitants in Hepatic Encephalopathy: A New Look at a Field in Continuous Evolution.J Clin Med. 2023 Feb 2;12(3):1187. doi: 10.3390/jcm12031187. J Clin Med. 2023. PMID: 36769836 Free PMC article. Review.
-
Recurrent Clostridioides difficile Infection (CDI) in Patients Treated With Vancomycin at Johns Hopkins Aramco Healthcare (JHAH), Dhahran, Saudi Arabia.Cureus. 2025 May 31;17(5):e85116. doi: 10.7759/cureus.85116. eCollection 2025 May. Cureus. 2025. PMID: 40599506 Free PMC article.
-
Fecal Microbiota Transplantation in Liver Cirrhosis.Biomedicines. 2023 Oct 30;11(11):2930. doi: 10.3390/biomedicines11112930. Biomedicines. 2023. PMID: 38001930 Free PMC article. Review.
References
-
- Role of gut microbiota in liver disease. Albhaisi SA, Bajaj JS, Sanyal AJ. Am J Physiol Gastrointest Liver Physiol. 2020;318:0–98. - PubMed
-
- Fecal microbiota transplantation in hepatic encephalopathy: a systematic review. Madsen M, Kimer N, Bendtsen F, Petersen AM. Scand J Gastroenterol. 2021;56:560–569. - PubMed
-
- Intestinal microbiota in liver disease. Haque TR, Barritt AS 4th. Best Pract Res Clin Gastroenterol. 2016;30:133–142. - PubMed
Publication types
LinkOut - more resources
Full Text Sources