Managing Manganese: The Role of Manganese Homeostasis in Streptococcal Pathogenesis
- PMID: 35800897
- PMCID: PMC9253540
- DOI: 10.3389/fcell.2022.921920
Managing Manganese: The Role of Manganese Homeostasis in Streptococcal Pathogenesis
Abstract
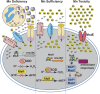
Pathogenic streptococci require manganese for survival in the host. In response to invading pathogens, the host recruits nutritional immune effectors at infection sites to withhold manganese from the pathogens and control bacterial growth. The manganese scarcity impairs several streptococcal processes including oxidative stress defenses, de novo DNA synthesis, bacterial survival, and virulence. Emerging evidence suggests that pathogens also encounter manganese toxicity during infection and manganese excess impacts streptococcal virulence by manganese mismetallation of non-cognate molecular targets involved in bacterial antioxidant defenses and cell division. To counter host-imposed manganese stress, the streptococcal species employ a sophisticated sensory system that tightly coordinates manganese stress-specific molecular strategies to negate host induced manganese stress and proliferate in the host. Here we review the molecular details of host-streptococcal interactions in the battle for manganese during infection and the significance of streptococcal effectors involved to bacterial pathophysiology.
Keywords: bacterial virulence; manganese homeostasis; manganese toxicity; manganese uptake; nutritional immunity; streptococcal infection.
Copyright © 2022 Aggarwal and Kumaraswami.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

