Sex differences in the diathetic effects of shift work schedules on circulating cytokine levels and pathological outcomes of ischemic stroke during middle age
- PMID: 35800977
- PMCID: PMC9253906
- DOI: 10.1016/j.nbscr.2022.100079
Sex differences in the diathetic effects of shift work schedules on circulating cytokine levels and pathological outcomes of ischemic stroke during middle age
Abstract
Shift work is associated with increased risk for vascular disease, including stroke- and cardiovascular-related mortality. However, evidence from these studies is inadequate to distinguish the effect of altered circadian rhythms in isolation from other risk factors for stroke associated with shift work (e.g., smoking, poor diet, lower socioeconomic status). Thus, the present study examined the diathetic effects of exposure to shifted LD cycles during early adulthood on circadian rhythmicity, inflammatory signaling and ischemic stroke pathology during middle age, when stroke risk is high and outcomes are more severe. Entrainment of circadian activity was stable in all animals maintained on a fixed light:dark 12:12 cycle but was severely disrupted during exposure to shifted LD cycles (12hr advance/5d). Following treatment, circadian entrainment in the shifted LD group was distinguished by increased daytime activity and decreased rhythm amplitude that persisted into middle-age. Circadian rhythm desynchronization in shifted LD males and females was accompanied by significant elevations in circulating levels of the inflammatory cytokine IL-17A and gut-derived inflammatory mediator lipopolysaccharide (LPS) during the post-treatment period. Middle-cerebral artery occlusion, 3 months after exposure to shifted LD cycles, resulted in greater post-stroke mortality in shifted LD females. In surviving subjects, sensorimotor performance, assessed 2- and 5-days post-stroke, was impaired in males of both treatment groups, whereas in females, recovery of function was observed in fixed but not shifted LD rats. Overall, these results indicate that early exposure to shifted LD cycles promotes an inflammatory phenotype that amplifies stroke impairments, specifically in females, later in life.
Keywords: Circadian rhythm dysregulation; IL-17A; Inflammation; Lipopolysaccharide; Middle cerebral artery; Stroke pathology.
© 2022 The Authors.
Conflict of interest statement
There are no potential competing interests (financial/personal) in which the authors’ professional judgment or objectivity was influenced with regard to the validity of research.
Figures



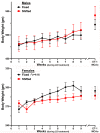
 ) or shifted (
) or shifted ( ) LD 12:12 cycles and immediately prior to ET-1-induced MCAo surgery. Simple linear regression analysis of LD treatment group differences indicates weight gain was significantly greater in fixed, than in shifted, LD females (*p = 0.03).
) LD 12:12 cycles and immediately prior to ET-1-induced MCAo surgery. Simple linear regression analysis of LD treatment group differences indicates weight gain was significantly greater in fixed, than in shifted, LD females (*p = 0.03).


Similar articles
-
Sex Differences in the Impact of Shift Work Schedules on Pathological Outcomes in an Animal Model of Ischemic Stroke.Endocrinology. 2016 Jul;157(7):2836-43. doi: 10.1210/en.2016-1130. Epub 2016 Jun 2. Endocrinology. 2016. PMID: 27254002 Free PMC article.
-
Shift work schedules alter immune cell regulation and accelerate cognitive impairment during aging.J Neuroinflammation. 2025 Jan 8;22(1):4. doi: 10.1186/s12974-024-03324-z. J Neuroinflammation. 2025. PMID: 39780172 Free PMC article.
-
Thermocyclic and photocyclic entrainment of circadian locomotor activity rhythms in sleepy lizards, Tiliqua rugosa.Chronobiol Int. 2009 Oct;26(7):1369-88. doi: 10.3109/07420520903412392. Chronobiol Int. 2009. PMID: 19916837
-
From circadian clock mechanism to sleep disorders and jet lag: Insights from a computational approach.Biochem Pharmacol. 2021 Sep;191:114482. doi: 10.1016/j.bcp.2021.114482. Epub 2021 Feb 20. Biochem Pharmacol. 2021. PMID: 33617843 Review.
-
Social influences on mammalian circadian rhythms: animal and human studies.Biol Rev Camb Philos Soc. 2004 Aug;79(3):533-56. doi: 10.1017/s1464793103006353. Biol Rev Camb Philos Soc. 2004. PMID: 15366762 Review.
Cited by
-
Night shift work was associated with functional outcomes in acute ischemic stroke patients treated with endovascular thrombectomy.Heliyon. 2024 Feb 10;10(4):e25916. doi: 10.1016/j.heliyon.2024.e25916. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38390161 Free PMC article.
-
Unhealthy lifestyle associated with increased risk of macro- and micro-vascular comorbidities in patients with long-duration type 2 diabetes: results from the Taiwan Diabetes Registry.Diabetol Metab Syndr. 2023 Mar 8;15(1):38. doi: 10.1186/s13098-023-01018-9. Diabetol Metab Syndr. 2023. PMID: 36890551 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

