A partial reduction of VDAC1 enhances mitophagy, autophagy, synaptic activities in a transgenic Tau mouse model
- PMID: 35801276
- PMCID: PMC9381918
- DOI: 10.1111/acel.13663
A partial reduction of VDAC1 enhances mitophagy, autophagy, synaptic activities in a transgenic Tau mouse model
Abstract
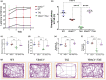
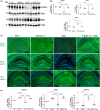
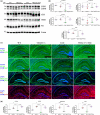
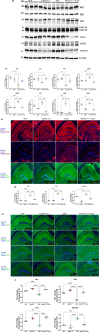
Alzheimer's disease (AD) is the most common cause of mental dementia in the aged population. AD is characterized by the progressive decline of memory and multiple cognitive functions, and changes in behavior and personality. Recent research has revealed age-dependent increased levels of VDAC1 in postmortem AD brains and cerebral cortices of APP, APPxPS1, and 3xAD.Tg mice. Further, we found abnormal interaction between VDAC1 and P-Tau in the AD brains, leading to mitochondrial structural and functional defects. Our current study aimed to understand the impact of a partial reduction of voltage-dependent anion channel 1 (VDAC1) protein on mitophagy/autophagy, mitochondrial and synaptic activities, and behavior changes in transgenic TAU mice in Alzheimer's disease. To determine if a partial reduction of VDAC1 reduces mitochondrial and synaptic toxicities in transgenic Tau (P301L) mice, we crossed heterozygote VDAC1 knockout (VDAC1+/- ) mice with TAU mice and generated double mutant (VDAC1+/- /TAU) mice. We assessed phenotypic behavior, protein levels of mitophagy, autophagy, synaptic, other key proteins, mitochondrial morphology, and dendritic spines in TAU mice relative to double mutant mice. Partial reduction of VDAC1 rescued the TAU-induced behavioral impairments such as motor coordination and exploratory behavioral changes, and learning and spatial memory impairments in VDAC1+/- /TAU mice. Protein levels of mitophagy, autophagy, and synaptic proteins were significantly increased in double mutant mice compared with TAU mice. In addition, dendritic spines were significantly increased; the mitochondrial number was significantly reduced, and mitochondrial length was increased in double mutant mice. Based on these observations, we conclude that reduced VDAC1 is beneficial in symptomatic-transgenic TAU mice.
Keywords: Alzheimer's disease; autophagy; hexokinases; mitochondria; mitochondrial biogenesis; mitophagy; oxidative stress; voltage-dependent anion channel 1.
© 2022 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Abu‐Hamad, S. , Zaid, H. , Israelson, A. , Nahon, E. , & Shoshan‐Barmatz, V. (2008). Hexokinase‐I protection against apoptotic cell death is mediated via interaction with the voltage‐dependent anion channel‐1: Mapping the site of binding. The Journal of Biological Chemistry, 283(19), 13482–13490. 10.1074/jbc.M708216200 - DOI - PubMed
-
- Azoulay‐Zohar, H. , Israelson, A. , Abu‐Hamad, S. , & Shoshan‐Barmatz, V. (2004). In self‐defence: Hexokinase promotes voltage‐dependent anion channel closure and prevents mitochondria‐mediated apoptotic cell death. The Biochemical Journal, 377(Pt 2), 347–355. 10.1042/BJ20031465 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

