FAPhigh α-SMAlow cancer-associated fibroblast-derived SLPI protein encapsulated in extracellular vesicles promotes ovarian cancer development via activation of PI3K/AKT and downstream signaling pathways
- PMID: 35801406
- PMCID: PMC9541539
- DOI: 10.1002/mc.23445
FAPhigh α-SMAlow cancer-associated fibroblast-derived SLPI protein encapsulated in extracellular vesicles promotes ovarian cancer development via activation of PI3K/AKT and downstream signaling pathways
Abstract
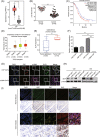
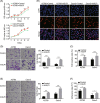
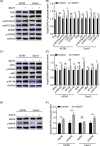
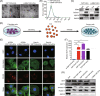
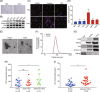
Ovarian cancer is the most lethal gynecological malignancy worldwide with high metastasis and poor prognosis rates. Cancer-associated fibroblasts (CAFs), a heterogeneous population of cells that constitutes a major component of the tumor microenvironment, secrete extracellular vesicles (EVs) loading with proteins, lipids, and RNAs to promote tumorigenesis. However, the specific roles of CAF-derived proteins contained in EVs in ovarian cancer remain poorly understood at present. Using the gene expression microarray analysis, we identified a list of dysregulated genes between the α-SMA+ CAF and FAP+ CAF subpopulations, from which secretory leukocyte protease inhibitor (SLPI) was chosen for further validation. Quantitative PCR, western blot, immunohistochemistry, and enzyme-linked immunosorbent assays were used to assess SLPI expression in ovarian cancer cells, tissues, CAFs, and EVs. Additionally, we evaluated the effects of exogenous SLPI on proliferation, migration, invasion, and adhesion of ovarian cancer cells in vitro. Our results showed SLPI protein was upregulated in CAFs, particularly in the FAPhigh α-SMAlow CAF subpopulation, and associated with increased tumor grade and decreased overall survival (OS). Importantly, CAF-derived SLPI protein could be encapsulated in EVs for delivery to ovarian cancer cells, thus facilitating cell proliferation, migration, invasion, and adhesion via activating the PI3K/AKT and downstream signaling pathways. Moreover, high plasma expression of SLPI encapsulated in EVs was closely correlated with tumor stage in ovarian cancer patients. Our collective results highlight an oncogenic role of plasma EV-encapsulated SLPI secreted by CAFs in tumor progression for the first time, supporting its potential utility as a prognostic biomarker of ovarian cancer.
Keywords: SLPI; cancer-associated fibroblasts; extracellular vesicles; ovarian cancer; tumor progression.
© 2022 The Authors. Molecular Carcinogenesis published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Extracellular vesicle-encapsulated microRNA-296-3p from cancer-associated fibroblasts promotes ovarian cancer development through regulation of the PTEN/AKT and SOCS6/STAT3 pathways.Cancer Sci. 2024 Jan;115(1):155-169. doi: 10.1111/cas.16014. Epub 2023 Nov 16. Cancer Sci. 2024. PMID: 37972389 Free PMC article.
-
CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells.Biosci Rep. 2017 Apr 10;37(2):BSR20160470. doi: 10.1042/BSR20160470. Print 2017 Apr 28. Biosci Rep. 2017. PMID: 28258248 Free PMC article.
-
Tumor-Derived Extracellular Vesicles Promote Activation of Carcinoma-Associated Fibroblasts and Facilitate Invasion and Metastasis of Ovarian Cancer by Carrying miR-630.Front Cell Dev Biol. 2021 Jun 30;9:652322. doi: 10.3389/fcell.2021.652322. eCollection 2021. Front Cell Dev Biol. 2021. Retraction in: Front Cell Dev Biol. 2024 Jun 14;12:1445367. doi: 10.3389/fcell.2024.1445367. PMID: 34277601 Free PMC article. Retracted.
-
The Role of Cancer-Associated Fibroblasts and Extracellular Vesicles in Tumorigenesis.Int J Mol Sci. 2020 Sep 17;21(18):6837. doi: 10.3390/ijms21186837. Int J Mol Sci. 2020. PMID: 32957712 Free PMC article. Review.
-
Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion.Cell Death Dis. 2022 Jan 18;13(1):64. doi: 10.1038/s41419-022-04510-8. Cell Death Dis. 2022. PMID: 35042862 Free PMC article. Review.
Cited by
-
The role of extracellular vesicles in the pathogenesis of gynecological cancer.Front Oncol. 2024 Sep 26;14:1477610. doi: 10.3389/fonc.2024.1477610. eCollection 2024. Front Oncol. 2024. PMID: 39391238 Free PMC article. Review.
-
Modeling Stromal Cells Inside the Tumor Microenvironment of Ovarian Cancer: In Vitro Generation of Cancer-Associated Fibroblast-Like Cells and Their Impact in a 3D Model.MedComm (2020). 2025 Apr 17;6(5):e70172. doi: 10.1002/mco2.70172. eCollection 2025 May. MedComm (2020). 2025. PMID: 40255916 Free PMC article.
-
Tissue-derived extracellular vesicles in cancer progression: mechanisms, roles, and potential applications.Cancer Metastasis Rev. 2024 Jun;43(2):575-595. doi: 10.1007/s10555-023-10147-6. Epub 2023 Oct 18. Cancer Metastasis Rev. 2024. PMID: 37851319 Review.
-
Applications of FAPI PET/CT in the diagnosis and treatment of breast and the most common gynecologic malignancies: a literature review.Front Oncol. 2024 Mar 5;14:1358070. doi: 10.3389/fonc.2024.1358070. eCollection 2024. Front Oncol. 2024. PMID: 38505595 Free PMC article. Review.
-
Recent advances in understanding the immune microenvironment in ovarian cancer.Front Immunol. 2024 Jun 5;15:1412328. doi: 10.3389/fimmu.2024.1412328. eCollection 2024. Front Immunol. 2024. PMID: 38903506 Free PMC article. Review.
References
-
- Ebell MH, Culp MB, Radke TJ. A systematic review of symptoms for the diagnosis of ovarian cancer. Am J Prev Med. 2016;50(3):384‐394. - PubMed
-
- Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4):280‐304. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7‐30. - PubMed
-
- Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316(8):1324‐1331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

