Reactive oxygen species activated by mitochondria-specific camptothecin prodrug for enhanced chemotherapy
- PMID: 35801419
- PMCID: PMC9589304
- DOI: 10.17305/bjbms.2022.7194
Reactive oxygen species activated by mitochondria-specific camptothecin prodrug for enhanced chemotherapy
Abstract
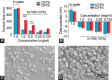
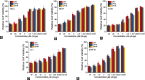
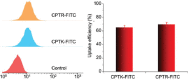
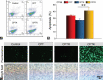
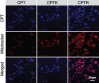
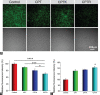
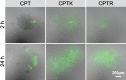
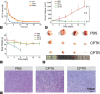
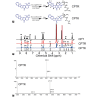
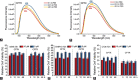
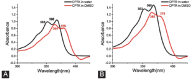
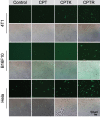
Camptothecin (CPT) has attracted much attention due to its potent antitumor activities. However, the undesirable physicochemical properties, including poor water-solubility, unstable lactone ring and severe adverse effects limit its further application. In this study, two water-soluble prodrugs, CPT-lysine (CPTK) and CPT-arginine (CPTR), were designed and synthesized by conjugating lysine or arginine with CPT, improving its solubility, pharmacokinetic properties and tumor penetration. Importantly, the introduction of arginine into CPTR contributed to the mitochondria-specific delivery, which increased mitochondrial reactive oxygen species (ROS) generation, induced mitochondria dysfunction and enhanced cell apoptosis and in vivo anti-cancer effect. This strategy is believed to hold great potential for organelle-specific synergistic anti-tumor therapy.
Conflict of interest statement
Conflicts of interest: The authors declare no conflicts of interest.
Figures

References
-
- Hertzberg RP, Caranfa MJ, Hecht SM. On the mechanism of topoisomerase-I inhibition by Camptothecin-evidence for binding to an enzyme DNA complex. Biochemistry. 1989;28(11):4629–38. https://doi.org/10.1021/bi00437a018. - PubMed
-
- Acevedo-Morantes CY, Acevedo-Morantes MT, Suleiman-Rosado D, Ramirez-Vick JE. Evaluation of the cytotoxic effect of camptothecin solid lipid nanoparticles on MCF7 cells. Drug Deliv. 2013;20(8):338–48. https://doi.org/10.3109/10717544.2013.834412. - PubMed
-
- Gao Y, Li LB, Zhai GX. Preparation and characterization of Pluronic/TPGS mixed micelles for solubilization camptothecin. Colloid Surface B. 2008;64(2):194–9. https://doi.org/10.1016/j.colsurfb.2008.01.021. - PubMed
-
- Fassberg J, Stella VJ. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogs. J Pharm Sci. 1992;81(7):676–84. https://doi.org/10.1002/jps.2600810718. - PubMed
-
- Mi ZH, Burke TG. Differential interactions of camptothecin lactone and carboxylate forms with human blood components. Biochemistry. 1994;33(34):10325–36. https://doi.org/10.1021/bi00200a013. - PubMed

