Design, synthesis and molecular docking of new fused 1 H-pyrroles, pyrrolo[3,2- d]pyrimidines and pyrrolo[3,2- e][1, 4]diazepine derivatives as potent EGFR/CDK2 inhibitors
- PMID: 35801486
- PMCID: PMC9272933
- DOI: 10.1080/14756366.2022.2096019
Design, synthesis and molecular docking of new fused 1 H-pyrroles, pyrrolo[3,2- d]pyrimidines and pyrrolo[3,2- e][1, 4]diazepine derivatives as potent EGFR/CDK2 inhibitors
Abstract
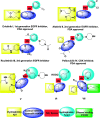
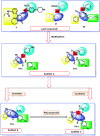
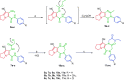
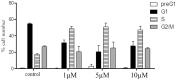
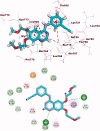
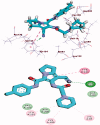
A new series of 1H-pyrrole (6a-c, 8a-c), pyrrolo[3,2-d]pyrimidines (9a-c) and pyrrolo[3,2-e][1, 4]diazepines (11a-c) were designed and synthesised. These compounds were designed to have the essential pharmacophoric features of EGFR Inhibitors, they have shown anticancer activities against HCT116, MCF-7 and Hep3B cancer cells with IC50 values ranging from 0.009 to 2.195 µM. IC50 value of doxorubicin is 0.008 µM, compounds 9a and 9c showed IC50 values of 0.011 and 0.009 µM respectively against HCT-116 cells. Compound 8b exerted broad-spectrum activity against all tested cell lines with an IC50 value less than 0.05 µM. Compound 8b was evaluated against a panel of kinases. This compound potently inhibited CDK2/Cyclin A1, DYRK3 and GSK3 alpha kinases with 10-23% compared to imatinib (1-10%). It has also arrested the cell cycle of MCF-7 cells at the S phase. Its antiproliferative activity was further augmented by molecular docking into the active sites of EGFR and CDK2 cyclin A1.
Keywords: 1H-pyrrole; 4]diazepine; Anticancer; CDK2 inhibitor; EGFR inhibitor; pyrrolo[3,2-d]pyrimidine; pyrrolo[3,2-e][1.
Conflict of interest statement
All authors of the above manuscript have not declared any conflict of interest.
Figures










References
-
- El-Metwally SA, Abou-El-Regal MM, Eissa IH, et al. . Discovery of thieno [2, 3-d] pyrimidine-based derivatives as potent VEGFR-2 kinase inhibitors and anti-cancer agents. Bioorg Chem 2021;112:104947. - PubMed
-
- World Health Organization, Cancer . Available from: https://www.who.int/news-room/fact-sheets/detail/cancer [last accessed 1 March 2020].
-
- El-Zahabi MA, Sakr H, El-Adl K, et al. . Design, synthesis, and biological evaluation of new challenging thalidomide analogs as potential anticancer immunomodulatory agents. Bioorg Chem 2020;104:104218. - PubMed
-
- Chabner BA, Roberts TG.. Chemotherapy and the war on cancer. Nat Rev Cancer 2005;5:65–72. - PubMed
-
- G, Krauss, N, Schönbrunner, J, Cooper Biochemistry of signal transduction and regulation. Weinheim: Wiley Online Library; 2003.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
