Outcomes following SARS-CoV-2 infection in individuals with and without solid organ transplantation-A Danish nationwide cohort study
- PMID: 35801493
- PMCID: PMC9349987
- DOI: 10.1111/ajt.17142
Outcomes following SARS-CoV-2 infection in individuals with and without solid organ transplantation-A Danish nationwide cohort study
Abstract
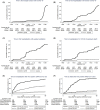
The risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, hospitalization and death, and the effects of SARS-CoV-2 vaccines in solid organ transplant recipients (SOTRs) is still debated. We performed a nationwide, population-based, matched cohort study, including all Danish SOTRs (n = 5184) and a matched cohort from the general population (n = 41 472). Cox regression analyses were used to calculate incidence rate ratios (IRRs). SOTRs had a slightly increased risk of SARS-CoV-2 infection and were vaccinated earlier than the general population. The overall risk of hospital contact with COVID-19, severe COVID-19, need for assisted respiration, and hospitalization followed by death was substantially higher in SOTRs (IRR: 32.8 95%CI [29.0-37.0], 9.2 [6.7-12.7], 12.5 [7.6-20.8], 12.4 [7.9-12.7]). The risk of hospitalization and death after SARS-CoV-2 infection decreased substantially in SOTRs after the emergence of the Omicron variant (IRR: 0.45 [0.37-0.56], 0.17 [0.09-0.30]). Three vaccinations reduced the risk of SARS-CoV-2 infection only marginally compared to two vaccinations, but SOTRs with three vaccinations had a lower risk of death (IRR: 022 [0.16-0.35]). We conclude that SOTRs have a risk of SARS-CoV-2 infection comparable to the general population, but substantially increased the risk of hospitalization and death following SARS-CoV-2 infection. A third vaccination only reduces the risk of SARS-CoV2 infection marginally, but SOTRs vaccinated 3 times have reduced mortality.
Keywords: clinical research/practice; infection and infectious agents - viral; infection and infectious agents - viral: SARS-CoV-2/COVID-19; infectious disease; solid organ transplantation.
© 2022 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures


Comment in
-
Treating comorbidity in solid organ transplant recipients as a confounder or a mediator of patient outcomes.Am J Transplant. 2023 Jan;23(1):156-157. doi: 10.1111/ajt.17201. Epub 2023 Jan 11. Am J Transplant. 2023. PMID: 36148606 No abstract available.
References
-
- World Health Organization. WHO Coronavirus (SARS-COV-2) dashboard 2021. Published 2021. Accessed March 12, 2022. https://covid19.who.int/.
-
- Karam S, Wali RK. Current state of immunosuppression: past, present, and future. Crit Rev Eukaryot Gene Expr. 2015;25(2):113–134. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

