COVID-19 and plasma cells: Is there long-lived protection?
- PMID: 35801537
- PMCID: PMC9350162
- DOI: 10.1111/imr.13115
COVID-19 and plasma cells: Is there long-lived protection?
Abstract
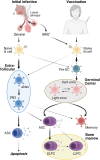
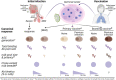
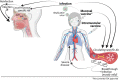
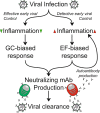
Infection with SARS-CoV-2, the etiology of the ongoing COVID-19 pandemic, has resulted in over 450 million cases with more than 6 million deaths worldwide, causing global disruptions since early 2020. Memory B cells and durable antibody protection from long-lived plasma cells (LLPC) are the mainstay of most effective vaccines. However, ending the pandemic has been hampered by the lack of long-lived immunity after infection or vaccination. Although immunizations offer protection from severe disease and hospitalization, breakthrough infections still occur, most likely due to new mutant viruses and the overall decline of neutralizing antibodies after 6 months. Here, we review the current knowledge of B cells, from extrafollicular to memory populations, with a focus on distinct plasma cell subsets, such as early-minted blood antibody-secreting cells and the bone marrow LLPC, and how these humoral compartments contribute to protection after SARS-CoV-2 infection and immunization.
Keywords: COVID-19; SARS-CoV-2; antibody secretion; antibody-secreting cell; long-lived plasma cell.
© 2022 The Authors. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
Competing interests: FEL is the founder of Micro‐Bplex, Inc. FEL serves on the scientific advisory board of Be Biopharma, is a recipient of grants from the BMGF and Genentech, Inc. FEL has also served as a consultant for Astra Zeneca. IS has consulted for GSK, Pfizer, Kayverna, Johnson & Johnson, Celgene, Bristol Myer Squibb, and Visterra. The other authors declare no conflicts of interest.
Figures
References
-
- Mobaraki PD, Wang C, Floridi A, Floridi A, Zaidi AK. Long‐Term Persistence of IgG Antibodies in recovered COVID‐19 individuals at 18 months and the impact of two‐dose BNT162b2 (Pfizer‐BioNTech) mRNA vaccination on the antibody response. 2022. https://www.medrxiv.org/content/10.1101/2022.01.18.22269349v1. Accessed February 27, 2022. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

