Phenylbutyrate modulates polyamine acetylase and ameliorates Snyder-Robinson syndrome in a Drosophila model and patient cells
- PMID: 35801587
- PMCID: PMC9310527
- DOI: 10.1172/jci.insight.158457
Phenylbutyrate modulates polyamine acetylase and ameliorates Snyder-Robinson syndrome in a Drosophila model and patient cells
Abstract
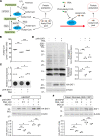
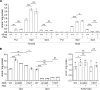
Polyamine dysregulation plays key roles in a broad range of human diseases from cancer to neurodegeneration. Snyder-Robinson syndrome (SRS) is the first known genetic disorder of the polyamine pathway, caused by X-linked recessive loss-of-function mutations in spermine synthase. In the Drosophila SRS model, altered spermidine/spermine balance has been associated with increased generation of ROS and aldehydes, consistent with elevated spermidine catabolism. These toxic byproducts cause mitochondrial and lysosomal dysfunction, which are also observed in cells from SRS patients. No efficient therapy is available. We explored the biochemical mechanism and discovered acetyl-CoA reduction and altered protein acetylation as potentially novel pathomechanisms of SRS. We repurposed the FDA-approved drug phenylbutyrate (PBA) to treat SRS using an in vivo Drosophila model and patient fibroblast cell models. PBA treatment significantly restored the function of mitochondria and autolysosomes and extended life span in vivo in the Drosophila SRS model. Treating fibroblasts of patients with SRS with PBA ameliorated autolysosome dysfunction. We further explored the mechanism of drug action and found that PBA downregulates the first and rate-limiting spermidine catabolic enzyme spermidine/spermine N1-acetyltransferase 1 (SAT1), reduces the production of toxic metabolites, and inhibits the reduction of the substrate acetyl-CoA. Taken together, we revealed PBA as a potential modulator of SAT1 and acetyl-CoA levels and propose PBA as a therapy for SRS and potentially other polyamine dysregulation-related diseases.
Keywords: Genetic diseases; Genetics; Lysosomes; Polyamines; Therapeutics.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

