An examination of the efficacy and safety of fenfluramine in adults, children, and adolescents with Dravet syndrome in a real-world practice setting: A report from the Fenfluramine European Early Access Program
- PMID: 35801621
- PMCID: PMC9712464
- DOI: 10.1002/epi4.12624
An examination of the efficacy and safety of fenfluramine in adults, children, and adolescents with Dravet syndrome in a real-world practice setting: A report from the Fenfluramine European Early Access Program
Abstract
Objective: To examine the efficacy and safety of fenfluramine in patients with Dravet syndrome (DS) in three age groups: <6, 6-17, and ≥18 years old, treated in a real-world setting.
Methods: Patients with DS were treated with fenfluramine in the European Union Early Access Program (EAP). Following a 28-day baseline period to establish the pretreatment monthly convulsive seizure frequency (MCSF), fenfluramine was started at a dose chosen by the treating physician and gradually titrated based on efficacy and tolerability up to a maximum of 0.7 mg/kg/day. Seizure incidence was recorded in a written diary, and adverse events (AEs) were reported at each patient visit. Cardiovascular safety was assessed by transthoracic echocardiography before treatment started and at least every 6 months thereafter.
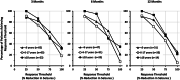
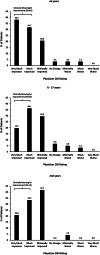
Results: A total of 149 patients have enrolled in the EAP and 63 were <6 years old, 62 were 6-17 years old, and 24 were ≥18 years old. After 3 months of treatment 62%, 53%, and 50% of patients demonstrated ≥75% reduction in MCSF in the <6, 6-17, and ≥18-year-old groups, respectively. This pattern of response was sustained through 12 months of treatment with 55%, 46%, and 80% of the <6, 6-17, and ≥18-year-old groups, respectively, experiencing a ≥75% reduction in MCSF. Most common AEs were loss of appetite (21%) and somnolence (16%). No valvular heart disease or pulmonary artery hypertension was observed.
Significance: The magnitude, consistency, and durability of the response to add-on fenfluramine is consistent across age groups in patients with Dravet syndrome.
Keywords: Dravet syndrome; clinical practice; fenfluramine; refractory epilepsy; seizures.
© 2022 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
RG reports research grants from Zogenix during conduct of the submitted work; speaking/consulting for Zogenix outside the submitted work; being an investigator for studies with Biocodex, UCB, Angelini, and Eisai Inc.; and serving on speaker/advisory boards for Biocodex, Novartis, BioMarin, and GW Pharma outside the submitted work. NS has served on scientific advisory boards for Zogenix, GW Pharma, BioMarin, Arvelle, Marinus, and Takeda; has received speaker honoraria from Zogenix, Eisai, BioMarin, Livanova, and Sanofi; and has served as an investigator for Zogenix, Marinus, BioMarin, UCB, and Roche. AA‐S received funding for research and educational activities from Zogenix, GW, UCB, Bial, Eisai, Sanofi, Neuraxpharm, and Arvelle. MP reports a research grant from Zogenix, Inc. FD reports research funding from Zogenix. TM reports research funding from Bial, Eisai, GW Pharma, UCB Pharma, and Zogenix. AG‐N reports personal fees or research grants from Arvelle Therapeutics, Bial, Biocodex, Eisai, Esteve, GW Pharma, PTC Therapeutics, Sanofi, Stoke, UCB, and Zogenix. TP reports research funding from Zogenix; and consulting and speaking for Desitin, Shire, Novartis, UCB Pharma, and Zogenix. SMZ reports research support from Epilepsy Research UK, Dravet Syndrome UK, and Zogenix; consultancy, advisory, and speaker support for GW Pharma, Encoded Therapeutics, Stoke Therapeutics, Eisai, UCB Pharma, Jaguar Gene Therapy, Arvelle, and Zogenix. AL is an employee of, and has ownership interest in, Zogenix. AGammaitoni is an employee of, and has ownership interest in, Zogenix, Inc. AS reports personal fees and grants from Angelini Pharma/Arvelle Therapeutics, Desitin Arzneimittel, Eisai, GW Pharmaceuticals, Marinus Pharma, UCB, UNEEG medical, and Zogenix. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
References
-
- Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl 2):3–9. - PubMed
-
- Cooper MS, McIntosh A, Crompton DE, McMahon JM, Schneider A, Farrell K, et al. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43–7. - PubMed
-
- Lagae L, Brambilla I, Mingorance A, Gibson E, Battersby A. Quality of life and comorbidities associated with Dravet syndrome severity: a multinational cohort survey. Dev Med Child Neurol. 2018;60(1):63–72. - PubMed
-
- Shmuely S, Sisodiya SM, Gunning WB, Sander JW, Thijs RD. Mortality in Dravet syndrome: a review. Epilepsy Behav. 2016;64(Pt A):69–74. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

